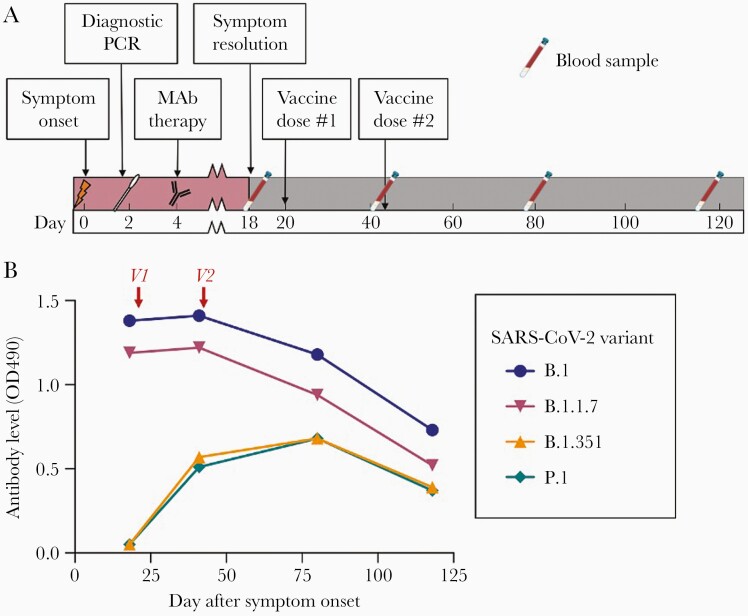
Figure 1.
A, Timeline of events. MAb, COVID-19-specific monoclonal antibody therapy (Bamlanivimab, Eli Lilly, Indianapolis, Indiana, USA); Vaccine, mRNA COVID-19 vaccine (BNT162b2, Pfizer-BioNTech, Philadelphia, Pennsylvania, USA). B, Antibody responses against receptor binding domain for common SARS-CoV-2 variants after infection/monoclonal antibody therapy and COVID-19 vaccine show increases in variant-specific antibodies after vaccination. V1 and V2, administration of first and second doses of mRNA COVID-19 vaccine (BNT162b2 OD490, RBD-specific antibody level measured by ELISA as optical density at 490 nm. Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; MAb, monoclonal antibody; PCR, polymerase chain reaction; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.