Summary
The hadal zone, mostly comprising of deep trenches and constituting of the deepest part of the world’s oceans, represents the least explored habitat but one of the last frontiers on our planet. The present scientific understanding of the hadal environment is still relatively rudimentary, particularly in comparison with that of shallower marine environments. In the last 30 years, continuous efforts have been launched in deepening our knowledge regarding the ecology of the hadal trench. However, the geological and environmental processes that potentially affect the sedimentary, geochemical and biological processes in hadal trenches have received less attention. Here, we review recent advances in the geology, biology, and environment of hadal trenches and offer a perspective of the hadal science involved therein. For the first time, we release high-definition images taken by a new full-ocean-depth manned submersible Fendouzhe that reveal novel species with an unexpectedly high density, outcrops of mantle and basaltic rocks, and anthropogenic pollutants at the deepest point of the world’s ocean. We advocate that the hydration of the hadal lithosphere is a driving force that influences a variety of sedimentary, geochemical, and biological processes in the hadal trench. Hadal lithosphere might host the Earth’s deepest subsurface microbial ecosystem. Future research, combined with technological advances and international cooperation, should focus on establishing the intrinsic linkage of the geology, biology, and environment of the hadal trenches.
Keywords: hadal trench, full-ocean-depth manned submersible, Mariana, subduction, marine pollution
Graphical abstract

Public summary
-
•
This paper provides a comprehensive review on hadal geology, environment, and biology, as well as potential interactions among them
-
•
For the first time, we release high-definition images taken by a new full-ocean-depth manned submersible Fendouzhe
-
•
The hydration of the hadal lithosphere is a driving force that influences a variety of sedimentary, geochemical, and biological processes in the hadal trench
-
•
The development of deep-sea technology and international cooperation will greatly promote the progress of hadal science
Introduction
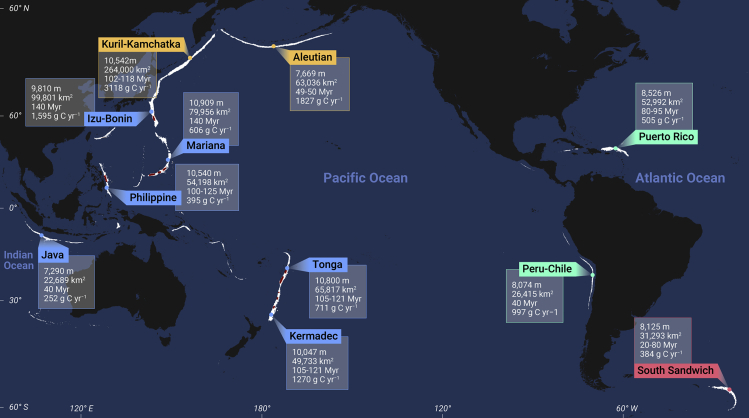
The hadal zone refers, in particular, to zones with 6,000 to 11,000 m water depth. These zones comprise only 1% of the world’s seafloor surface area but are key hinge points of the Earth system.1,2 Although other geological units such as hadal troughs in deep-sea basins and trench faults nearby mid-ocean ridges also fall within this range of depth, they are almost exclusively confined to the ocean’s 37 deep-sea trenches, the so-called hadal trenches (Figure 1). Tectonically, they are formed in subduction zones where old, cold subducting plates plunge beneath young, soft overriding plates, creating long but narrow topographic V-shaped depressions.3 The tremendous depths that mark fundamental breaks in the Earth’s lithosphere are controlled by several factors, including the sediment flux, the age of the incoming lithosphere, the convergence rate, dip of the subducted oceanic plate, and the width of the sinking plate.2,4 The nine deepest hadal trenches are located along the western arc of the Pacific Ocean. Among them, the Philippine, Mariana, Tonga, Kermadec, and Kuril-Kamchatka Trenches are deeper than 10,000 m.
Figure 1.
The distribution of hadal trenches over the world’s ocean
The map was generated using the ETOPO1 database and Generic Mapping Tools software. White color shows areas with water depth greater than 6,000 m. Dark red color highlights areas with water depths great than 9,000 m in hadal trenches, which are exclusively located in the Pacific Ocean. Values in small boxes show the maximum depth, the total area, the age of the subduction lithosphere, and the total flux of particulate organic carbon in each hadal trench.2,5,6 Yellow, blue, green, and red dots represent four different clusters of hadal trenches, which are grouped on how geographically distant each hadal area is from all other hadal areas.7
In the early stage of hadal trench research, Danish and Soviet scientists have made tremendous contributions.8,9 Hadal trench exploration culminated in 1960 when Jacques Piccard and Donald Walsh reached the bottom of the Challenger Deep by the manned bathyscaph Trieste. With the breakthrough in hadal technologies in recent years, hadal trenches are becoming one of the most interesting subjects in the field of marine science. In March 2012, James Cameron piloted the manned submersible Deepsea Challenger for scientific exploration to the Challenger Deep. More importantly, several hadal research plans have been funded in the United States, Japan, the UK, France, New Zealand, and China. For instance, the Hadal Ecosystems Studies Program, supported by the United States, is a collaborative program aimed at pursuing the foremost questions in hadal ecosystem science with the help of the full-ocean-depth unmanned submersible Nereus and imaging landers. A collaboration between the UK and Japan, the Hadal Environment and Educational Program, deployed free-fall-baited imaging landers at hadal depths to provide more insight into life in the hadal trenches.10 A 5-year program, the Hadal Science and Technology Program (HADSTEP), was also funded by Chinese Academy of Sciences in 2014. This program aimed to enhance the general understanding of the geological, environmental, and life processes in the deepest part of the Earth’s surface through developing full-ocean-depth technologies. In addition, using the manned submersible Limiting Factor, the Five Deeps Expedition was conducted in 2018–2019 and 39 dives were completed at the deepest points in each of the Earth’s five oceans. More recently, in 2020, China successfully conducted a sea trial for its full-ocean-depth manned submersible Fendouzhe, which was financed by the Ministry of Science and Technology of China. Thirteen dives were completed at the Challenge Deep of the southern Mariana Trench, eight of which exceeded 10,000 m, with a maximum dive depth of 10,909 m. High-definition images and high-quality biological and geological samples were obtained during the diving cruise. The rapid development of full-ocean technologies has provided the impetus for hadal exploration all over the world.
In the past 50 years, we have learned more about the ecology of the hadal trench than we did in all the preceding time periods. Far from being devoid of life as originally envisaged, we are now aware that hadal trenches host a diversity of hadal life with a high degree of endemism and high density.1,8 Evidence for hadal trench microbes that grow best at elevated hydrostatic pressure has fostered the development of an unusual branch of microbiology, high-pressure microbiology.11,12 To date, many piezophiles and piezotolerant bacteria, whose phenotypical and genomic features are distinct from those of the close relatives obtained from shallower environments, have been isolated from hadal environments.13 It was reported that the microbial communities in hadal water are enriched in potentially heterotrophic microbial populations, while those in overlying abyssal water are dominated by potentially chemolithotrophic populations.14 The hadal sediments harbor microbial communities with high cell abundance and high metabolic activity, which are largely related to the degradation of organic matter.14,15 The microbial community compositions and biogeographic diversification of microbial populations may be regulated by the trench geomorphology.15 A lot of efforts in hadal fauna have also been made to determine the composition, distribution, speciation and adaptation mechanism; and the role of pressure, food supply, physiology, depth, and seafloor topography on hadal fauna.1,16,17 Fifty-eight percent of the hadal fauna recovered are known to be endemic and survive in the hadal environment exclusively.18,19 The high hydrostatic pressure, the lack of light, and the remoteness from surface-derived food supply creates hostile living conditions for hadal life that are adapted to survive there. Recent studies have raised and partly answered highly interesting and speculative questions about the origin and evolution of hadal life.1,20
Compared to hadal ecology, our knowledge about hadal geology still remains rather deficient. Current knowledge about hadal geology is largely derived from geophysical techniques such as multibeam bathymetry and multichannel seismic reflection.21,22 Those methods are powerful to provide the regional-scale information on the tectonics and structural geology of the subduction zone, but usually lack the spatial resolution needed for fine-scale petrological research of the hadal trench. For example, although hydration of lithosphere is known to occur on the overriding plate and incoming plate of hadal trenches according to seismic reflection imaging,22,23 we know little about the major rock types that outcrop on the hadal seafloor, their degree of metamorphism, and their geological impacts on the hadal ecology. In addition, fluids released from the seafloor of the hadal trench still remain mysterious. Fluid discharges associated with hydration of mantle lithosphere in the shallowest parts of the hadal trench are expected to occur along the trench axes, but these have not been observed to date.2 At present, direct geological observation and rock samples retrieved from the hadal depths are scanty due to limited technical capabilities.
Except for extremely high hydrostatic pressure, the physical and chemical characteristics of the hadal trench, such as temperature, salinity, and concentrations of O2, nitrate, and other major geochemical components, differ little to those found at shallow depths.1,14,24,25 However, the V-shaped topography of the hadal trench, which is liable to trap the particulate matter delivered from natural sedimentation, ocean circulation, and turbidity currents triggered by earthquakes,26, 27, 28, 29, 30 makes the bottom of the hadal trench a unique sedimentary environment featuring a higher rate of organic carbon accumulation, elevated microbial activity, and higher benthic biomass compared with surrounding abyssal plains.26, 27, 28, 29, 30 Elevated diagenetic activity has been observed in the hadal environment; however, the anaerobic pathways and rates of organic matter diagenesis still need to be quantified.31,32 Another important advancement is the notion that the hadal environment has been highly contaminated by anthropogenic pollutants such as microplastics, persistent organic pollutants (POPs), mercury, and other toxic heavy elements.33,34 Hadal trenches have been regarded as repositories for pollutant amplification, both biological and topographically, which occurs regardless of the sources of these pollutants.33,35 Such contamination will have ecological and toxic effects, long term or ephemeral, depending on the scale of impact, but the ecological effects of these pollutants in hadal fauna are still less understood.
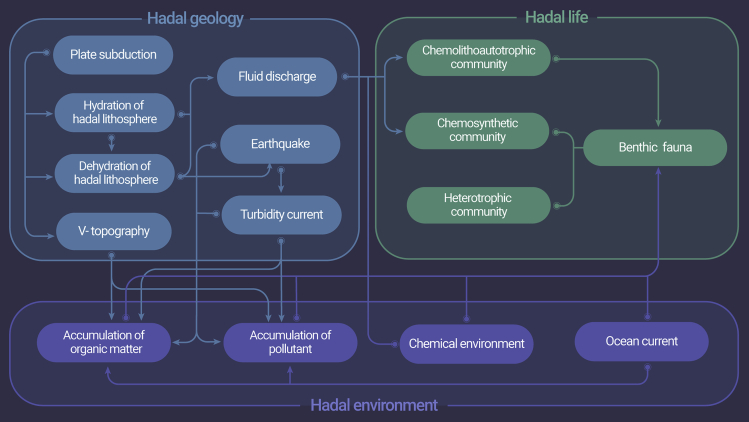
Here, as an attempt to integrate available knowledge, especially that obtained in the last 10 years, we provide a comprehensive review on hadal geology, hadal environment, and hadal biology. Logical links of sections and subsections are shown in Figure 2. Meanwhile, we release the high-definition images taken by the Chinese full-ocean-depth manned submersible Fendouzhe during its sea trial, which revealed novel hadal phenomena, as a significant supplement to the current hadal database.
Figure 2.
Logical links of sections and subsections showing potential interactions among the geology, life, and environment in the hadal trench
Hadal lithosphere, associated fluids, and life activities
Hydration of hadal lithosphere
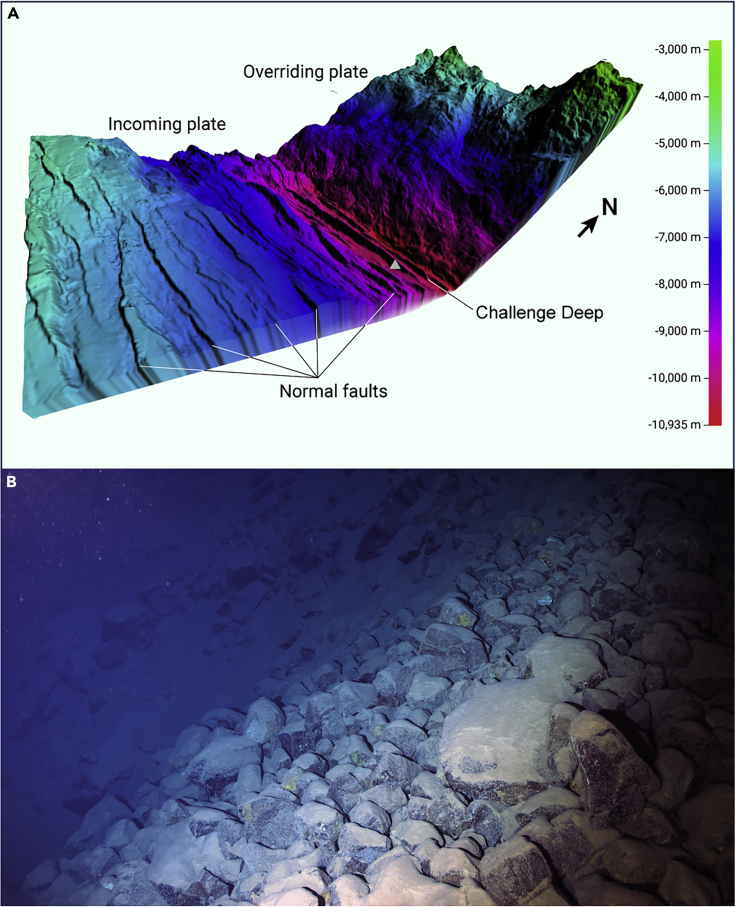
The water carried with the incoming plate into the deep lithosphere beneath the hadal trench affects the hydration and rheology of the lithosphere of the subduction zone. Although there remains considerable uncertainty regarding how much water is stored within the crust and mantle of the incoming plate, growing evidence suggests that much of the hydration of the incoming plate occurs at the trench slope right before subduction.22,23,36 This is because the bending of the incoming plate between the trench axis and outer rise area in response to the plate’s flexure is vigorous, creating numerous fractures, bending-related normal faults, and forming horst and graben structures (Figure 3). This mechanical process modifies the porosity and permeability structure of incoming lithosphere and provides pathways for fluids to enter through the crust and mantle. In addition, the bending of the incoming plate can generate subhydrostatic or even negative pressure gradients along the faults, promoting fluid flow to depth,37 leading to lithosphere hydration by the serpentinization of upper mantle rocks22 and, perhaps, of crustal rocks.37,38 A well-known example of the hydration of the incoming lithosphere comes from the Middle America Trench, where active bending-related faulting across the entire ocean trench slope penetrates at least 20 km into the plate, promoting the deep percolation of water and hydration of the crust and upper mantle.22,39,40 The hydration of upper mantle rock has also been documented below the Moho of the subducting slab at the northern central Chile,41 central Chile,23 southernmost Mariana,42 and Mariana43 trenches, as shown by a notable reduction in compressional mantle velocities, with a penetration depth of 1–2 km,22 and even 5–7 km, into the mantle.41,44,45
Figure 3.
Bending-related normal faults and fracture zones in the southern Mariana Trench
(A) Topographic map showing abundant bending-related normal faults occurring on the incoming plate of the southern Mariana Trench. The map was generated using multibeam bathymetric data collected by Kongsberg EM 124.
(B) Mélange (grey triangle in A) associated with a bending-related normal fault on the south slope of the Challenge Deep. The photograph was taken by the camera of Fendouzhe at a depth of 10,835 m.
The mantle wedge in the overriding plate of the subduction zone is also a very important and frequent site for the hydration of mantle rocks via serpentinization, as revealed by reduced seismic wave velocities and an enhanced VP/VS ratio.46,47 In the intra-oceanic subduction zone in the Pacific Ocean, the serpentinization of the mantle wedge has been widely identified at depths of 10–20 km, such as beneath the Izu-Bonin Trench,48 Mariana Trench,42,49 and Tonga Trench.41 The water required for mantle wedge serpentinization has been suggested to be derived from serpentinites by metamorphic dehydration reactions in the subducted oceanic crust,50 the devolatilization of subducted marine sediments,51 and altered oceanic basalts.52 Upward and downward fluid circulation is predominantly controlled by fracturing in the upper crust and mantle wedge, as well as by plastic deformation along the plate interface.53 The episodic release of fluids from the subducting slab along the fault structure could lead to the formation of serpentinite mud volcanoes in the forearc region above the hadal trench.4,54,55
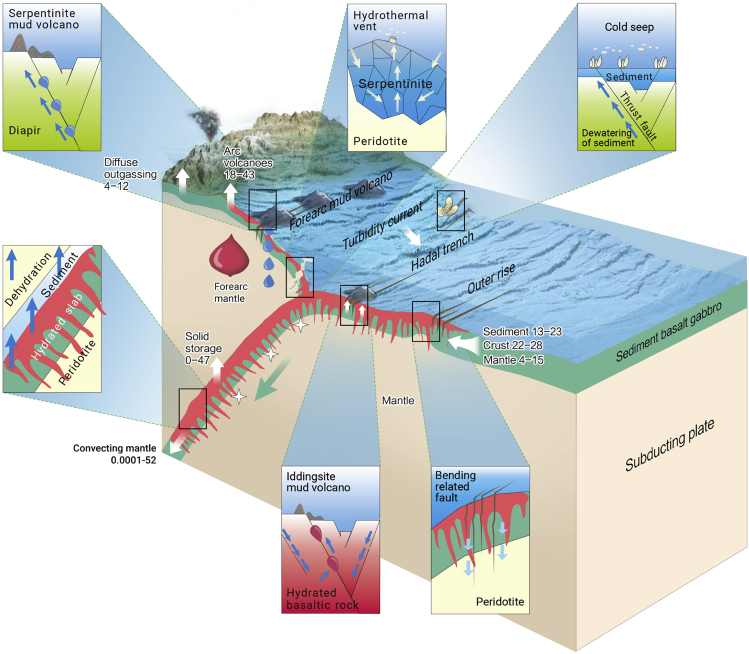
Growing evidence4,47,56,57 has shown that the hydration of the incoming plate, subsequent dehydration of the incoming slab, and hydration of the mantle wedge of the overriding plate have profound impacts on sedimentary, geochemical, and biological processes in the hadal trench (Figure 4): (1) the hydration and dehydration of the hadal lithosphere could support fluid discharge and fuel chemolithoautotrophic life on the hadal seafloor and within the hadal lithosphere,58, 59, 60 (2) the dehydration of the incoming plate could trigger earthquakes that substantially influence the sedimentary flux and organic carbon supply to the hadal trench,61,62 and (3) the hydration/alteration-derived sediments could increase the supply of carbon, iron, and other elements to the hadal trench and influence biogeochemical cycling therein.63
Figure 4.
Geological background of the hadal trench in the plate tectonic model
This model integrates available information on the hydration of the hadal lithosphere37,56, the fluid activity,55,59 and the carbon flux64 in the subduction zone. The green arrow indicates relative plate motion. Red spikes show the hydration of the oceanic lithosphere, including the basaltic and gabbroic crust and upper mantle. Blue and yellow arrows depict fluid ingress or expulsion. Magma generated in the forearc mantle is shown in purplish red. Stars represent seismic zones. White arrows show major carbon fluxes in the subduction zone. The unit of values is million tonnes of carbon (Mt C) per year. Key geological processes that occur on, beneath, and above the seafloor of the hadal trench are highlighted in small boxes.
Hydration of mantle lithosphere likely supporting fluid discharge
It is well known that hydration of mantle rocks can drive the serpentinite-hosted hydrothermal system at the deep-sea floor, which vents warm (40–90°C), high-pH (9–11) fluids enriched in H2 and CH4 that fuel chemolithotrophic life.65,66 In the forearc region of the Marina subduction zone, fluids from the incoming plate hydrate the forearc mantle and enable serpentinite muds to rise along faults to the seafloor and form serpentinite mud volcanoes.4,55 However, the serpentinite-hosted hydrothermal vent has not been discovered in the hadal trench so far. Could the hydration of forearc mantle rocks in the subduction zone also lead to the formation of serpentinite-hosted hydrothermal systems at the hadal depth?
The exposure of peridotite rocks that are expelled from the deep lithosphere at the seafloor of hadal trenches provides the prerequisite for this possibility. Michibayashi et al.67 reported that peridotite samples were recovered at 6,469–5,957 m along the eastward-facing slope of the West Santa Rosa Bank fault, and some of those samples exhibited high serpentinization. Reagan et al.68 reported on peridotite samples recovered from the Mariana forearc region at depths of 6,350 to 5,800 m during a Shinkai 6500 dive. Serpentinite and peridotite were also observed and recovered at a depth of 6,413 m on the inner trench slope of the Yap Trench during a Jiaolong dive.69 Interestingly, serpentinized peridotite and vesicomyid clams were discovered occurring together at a depth of 5,861 to 5,550 m at the Shinkai Seep Field on a scarp of the inner trench slope of the southern Mariana Trench during a Shinkai 6500 dive.57 This serpentinite-hosted ecosystem is supported by fault-controlled fluid pathways connected to the decollement of the subducting slab and is nourished by alkaline hydrothermal fluids with a high pH, low sulfate, and low magnesium.70 Putative chemolithoautotrophic microbial communities are also suggested to constitute the filamentous structures covering the outcrop of basement rocks at a 10.7 km depth in the Sirena Deep region of the Mariana Trench.71 Furthermore, during the Fendouzhe dive in 2020, extensive exposures of serpentinite rocks were observed on the northern slope of the Challenge Deep region of the Mariana Trench. It is expected that serpentinite-hosted hydrothermal systems will one day be discovered in the deepest part of the world’s ocean.
Hydration of basaltic lithosphere supporting fluid discharge
Compared with the hydration of ultramafic mantle rocks, the hydration of upper basaltic crust and its impact on the hadal environment and ecosystem have received less attention. A new type of fluid discharge at depths of 5,700–6,200 m was recently reported on the old and highly tectonized portions of the incoming plate of the southern Mariana Trench.59 The work by Du et al.59 provides direct evidence that the structural deformation of the incoming plate could substantially influence the hydration of upper basaltic crust, which fosters chemical exchange between the upper ocean crust and seawater in a new way.43 These newly discovered fluid discharge fields are exhibited by small-scale mud volcanoes and pockmarks in association with varied altered mafic rocks that crop out on the trench slope and are mainly composed of iddingsite.59 Chemically and physically, this phenomenon differs from the serpentine mud volcano formation associated with the shallower Mariana forearc region.55 Although the alteration reaction converting basaltic minerals to iddingsite is still not well understood in quantitative terms, a connection between iddingsitization and H2 generation during alteration processes has been proposed.72,73 Ferrous iron minerals in basaltic rocks, such as olivine and pyroxene, could react with water, producing iddingsite and generating H2. The general reaction can be expressed as 2(FeO) + H2O → Fe2O3 + H2. The H2 generated via iddingsitization may support rock-hosted, H2-based microbial ecosystems that thrive in old oceanic crusts of the subduction zone.74,75
Like iddingsitization, other very-low-grade metamorphisms related to the hydration of basaltic rocks also widely occur in the upper part of the oceanic crust, producing zeolite-facies metamorphic rocks that could be extruded on the seafloor of the hadal trench. A hypothesis that subduction zone zeolite-facies rock hosts Earth’s deepest subsurface microbial ecosystems is proposed.60 This is based on the fact that (1) zeolite-facies metamorphism starts at temperatures of approximately 40°C, well within the tolerance of life, and (2) zeolite-facies metamorphism can extend up to 14 km into the ocean crust of the subduction zone at temperatures below 121°C. To test this hypothesis, Peng et al.60 recovered zeolite-facies metamorphic rocks from the southern Mariana Trench at a water depth of 5,500 to 6,800 m using the manned submersible Jiaolong. Dense carbonaceous aggregates and filaments, which result from the past activity of chemolithoautotrophs, were observed to be preserved in those metamorphic rocks. This finding highlights that fluid-rock reactions, during the hydration of the upper basaltic crust, may contribute to biomass production within the biotope,59 and implies that the deep-subsurface biosphere on Earth may be far deeper than the basalt-hosted deep biosphere76, 77, 78 and ultramafic rock-hosted deep biosphere.79 The low-grade metamorphic ocean crust in the hadal trench may represent previously unexplored incubators for chemolithoautotrophic life on Earth and could have significant implications for the global carbon flux and budget.
Faults controlling the distribution of cold seeps
Cold seeps occur along active margins driven by convergence between oceanic and continental plates and extend from continental slopes to hadal trenches. Unlike serpentinite-hosted hydrothermal vents in the hadal trench, they result from tectonic fluid expulsion by the dewatering of sediments that occurs in response to lateral compression by plate movement.80 Over long periods of accretion, the landward slope of the hadal trench develops a series of thrust faults that provide pathways for fluid discharge and control the distribution of cold seeps on the seafloor. Therefore, the geometric arrangement of cold seeps and associated communities can provide a kinematic indicator of the distribution pattern of faults and relative plate motions. Using the manned submersible Shinkai 6500, Ogawa et al.58 first observed the distribution of Calyptogena phaseoliformis clam colonies in right-stepping en echelon patterns related to the plate motion along the escarpment in the northern Japan Trench at 6,437–6,274 m. Seep fluid outflows along fractures are associated with thrust faults at the seafloor and are characterized by high CH4 and H2S concentrations. These chemical species supply energy to the food chain through bacteria utilizing CH4 and H2S. Fujikura et al.81 also reported the discovery of a dense community of the thyasirid bivalves Maorithyas hadalis by the remotely operated vehicle Kaiko at a depth of 7,326 m near the bottom of the Japan Trench. This chemosynthesis-based community was the deepest cold-seep-based community discovered from the hadal trench.81
Sedimentary environment of the hadal trench
Sources of organic matter
Uncovering sources of organic matter in the hadal trench has attracted extensive attention. It has been speculated that the supply of nutrition food in the hadal trench largely relies on the flux of fresh, organic-rich particulate matter14,27 and the input of decaying biota and carcasses from the upper ocean.61 Several studies have indicated that the organic matter contents in the trenches increase from the abyssal depth to the hadal zone (e.g., Eloe et al.82 and Jamieson83).
In a broad sense, organic matter in trench sediments derives from either marine or terrestrial sources. Their relative proportion depend mainly on the surface ocean productivity, the water depth, and their geographic locations. For instance, the sediment organic materials in the Japan,84 Yap,85 and Mariana Trenches86 are predominantly derived from marine phytoplankton, with negligible terrestrial organic matter, because they are located far away from large land masses. By contrast, the Izu-Bonin and New Britain Trenches, which are located in relative proximity to large islands, showed a high supply of allochthonous terrigenous organic material.29,87
Besides terrigenous- and marine-phytoplankton-derived organic materials, growing evidence indicates that chemoautotrophic carbon fixation occurring within the hadal trench is likely to be another important source of organic carbon in the trench. As reviewed in previous sections “hydration of mantle lithosphere likely supporting fluid discharge, “hydration of basaltic lithosphere supporting fluid discharge”, and “faults controlling the distribution of cold seeps”, chemosynthetic communities that are fueled by the serpentinization of mantle rocks, iddingsitization of basaltic rocks, and cold-seep fluids could substantially contribute organic carbon to the hadal trench.57,59,60,88,89 The compositional and spatial distribution patterns of glycerol dialkyl glycerol tetraethers (GDGTs) can be used as a valuable molecular indicator for organic carbon sources in the hadal trench.90, 91, 92 Recently, a distinctly high relative abundance of bacterial GDGTs produced in situ (branched and isoprenoid tetraether [BIT] = 0.13–0.80 and Rb/i = 0.08–11.37) were observed at two sediment cores of both sides of trench walls in the southern Mariana Trench.93 Notably, the result is similar to that for the altered rocks reported by Peng et al.,60 which showed that the BIT values fell within a range of 0.42 to 0.97. Simultaneously, the GDGT-0 to crenarchaeol ratio in the altered rocks ranges from 1.4 to 59.5, indicating a methanogenic source. These studies suggest that the contribution of chemosynthetic carbon fixation in the hadal trench should not be ignored; it might be an important supplement to the organic carbon pool in the hadal environment.
Earthquakes influencing sediment flux
The hydration and subsequent dehydration of oceanic lithosphere is an important internal force that drives geological and sedimentary processes in the hadal trench. Water released by the dehydration of oceanic upper mantle could trigger intraslab intermediate-depth earthquakes at depths of 50–300 km beneath the seafloor of the hadal trench.50,94,95 The strong shaking of the ground in earthquakes could drive turbidity currents, landslides, and tsunamis that strongly affect the sedimentary processes and organic carbon fluxes on the bottom of the trench. Near-bottom in situ video observations and sediment cores clearly document the remobilization of surficial sediment triggered by these earthquakes.61,62,96 Oguri et al.61 observed that benthic megafauna were absent, but dead organisms were present along the axes of the Japan Trench after the 2011 Tohoku-Oki earthquake. Their findings suggest that both the burial of organisms and the episodic delivery of organic matter to greater water depths commonly occur in trenches. Bao et al.97 revealed that hadal sedimentation was interrupted by the episodic deposition of pre-aged organic carbon triggered by large earthquakes, such as the 2011 Tohoku-Oki earthquake, in the Japan Trench. Kioka et al.98 found that surficial sediment remobilization is a predominant remobilization process that partly initiates deeper sediment remobilization downslope during strong earthquakes in the Japan Trench. Furthermore, Kioka et al.98 estimated that the Tohoku-Oki earthquake in 2011 could have delivered >1 Tg of organic carbon to the Japan Trench, which is comparable with the high carbon fluxes described for other Earth system processes. Evaluating how much organic carbon in the hadal environment is supplied by earthquakes in the present and the geological past will be important for understanding earthquakes’ role in the benthic ecosystem, carbon cycling, and carbon dynamics in the deep sea.
Elevated diagenetic activity of organic matter
As described in section 3.2, tectonically triggered mass-wasting events transport large amounts of sedimentary matter from the adjacent margin to the hadal trench.61,97 The sediments in the hadal trench are generally characterized by a higher rate of organic carbon accumulation, elevated microbial activity, and higher benthic biomass compared with surrounding abyssal plains (e.g., Danovaro et al.,26 Glud et al.,27 Leduc et al.,28 Luo et al.29,30). The funnel-like shape of the hadal trench easily traps settling particulate matter delivered from natural sedimentation, ocean circulation, and submarine turbidity currents.26,27,85,99 Therefore, they are considered to be potential depocenters for sediments and organic matter,26,27,86,100,101 accompanying the intensified diagenetic activity existing in this extreme environment.
Quantifying the pathways and rates of organic matter diagenesis in sediments is essential for understanding global element cycles, as it is directly or indirectly coupled to almost all biogeochemical processes. A few studies have discussed organic matter degradation in hadal sedimentary environments. An example is the study of Glud et al.,27 who first studied microbial activity in sediments at the Challenger Deep of the Mariana Trench (11,000 m) and a nearby 6,000-m deep site with an autonomous micro-profiling system to determine the benthic O2consumption rates. They showed that hadal sediments possess higher organic carbon contents and intensified diagenetic activity along the trench axis than in the adjacent abyssal plain. Further studies quantified the in situ benthic O2 consumption rates in sediments from the different hadal trenches underlying mesotrophic or oligotrophic surface waters, such as the Izu-Bonin Trench, Tonga Trench,102 Mussau Trench, and New Britain Trench.29
These findings complement those of Glud et al.,27 that hadal trenches host elevated diagenetic activity in the central trench, underpinning the importance of hadal ecosystems for deep-sea carbon cycling. However, these studies solely focused on aerobic respiration in organic matter oxidation. Microbial and isotopic studies have implied the occurrence of anaerobic diagenetic processes (such as denitrification),103,104 but few studies have directly measured them yet. Based on the geochemical characteristics of pore water, Liu and Peng105 emphasized the importance of denitrification in sedimentary diagenesis along a depth transect of the Mariana Trench (water depths from 5,500 to 10,255 m). Hiraoka et al.15 reported that NO3– concentrations drastically decreased with sediment depth to less than 5 μM above 30 cm below sea floor at the Japan and Izu-Ogasawara Trenches, suggesting that microbial nitrate reduction was occurring in hadal sediments. Based on geochemical and biomolecular analysis, Thumdrup et al.32 demonstrated that denitrification and anammox are major sinks for fixed nitrogen in hadal sediments along the axis of the Kermadec and Atacama Trenches. Moreover, the pore-water profiles and onboard incubations of the Kermadec and Atacama Trench sediments revealed variable but substantial manganese and iron redox cycling and sulfate respiration, further implying significant contributions from anaerobic diagenesis at these trench sites.31
Microbial communities in the hadal trench
Only a few studies have been carried out to characterize the microbiology of the water column and sediment in hadal trenches, largely due to the difficulties in sampling. These investigations to date have mainly focused on the Mariana,14 Japan,106 Kermadec,107 New Britain,108 and Puerto Rico Trenches.109 Compared with upper abyssal oceans, hadal zones possess comparable environmental factors such as temperature, salinity, dissolved O2, and regular nutrients. However, due to the tremendous depth, V-shaped geomorphology, and tectonic subduction, there are a series of environmental particularities, including an extremely high hydrostatic pressure, hydrotopographical isolation, and subduction-linked physical and chemical features, which are believed to contribute to the formation of a unique hadal biosphere in the deepest ocean on Earth.1,14 Despite the harsh environmental conditions, hadal trenches have been revealed to accommodate a diverse and active microbiome in the water and sediments.110,111
Studies suggest that there is a clear shift in microbial community compositions from bathyal and abyssal waters to deep hadal zones. Moreover, potential chemolithotrophs are less abundant in hadal waters, while the proportions of heterotrophic lineages are enhanced.14 Eloe et al.82 analyzed the metagenomic characteristics of microbial assemblages in water samples taken at a 6,000-m depth from the Puerto Rico Trench (PRT). It was found that microbial members from the β-Proteobacteria, Bacteroidetes, and Planctomycetes phyla were remarkably abundant compared to those in other pelagic environments. In addition, a large number of functional genes encoding for outer membrane porins, sulfatases for the degradation of sulfated polysaccharides, and glyoxylate and dicarboxylate metabolism were retrieved from the PRT hadal waters, indicating the prevalence of heterotrophic catabolic processes.109 Recently, microbial communities inhabiting the waters of the Challenger Deep in the Mariana Trench were profiled along the vertical depths, from the surface down to the bottom at 10,927 m.14 The microbial cell abundances were relatively constant at ∼ 6×103 cells/mL in hadal waters, but showed a gradual, slight decline with increasing depth. An analysis of the community structures based on the small subunit ribosomal RNA (SSU rRNA) gene revealed an unprecedented hadal biosphere in which potential heterotrophs, including the SAR406 clade, Bacteroidetes, and γ-proteobacterial Halomonas and Pseudomonas, dominated the trench communities below 6,000 m. It was further hypothesized that the unique hadal biosphere in the Mariana Trench was principally driven by endogenous organic matter, which was chiefly controlled by the trench’s extraordinary geomorphology.14,83 More recently, Tarn et al.111 further characterized the microbiome within the bottom waters of the Challenger and Sirena Deeps in the Mariana Trench. They also found that heterotrophic lineages dominated the bacterial communities of both hadal regions, which were distinctive from other deep-sea habitats. These hadal-enriched bacterial taxa were mainly dispersed within Marinimicrobia, ε-Proteobacteria, and Gemmatimonadetes, consistent with previous hadal datasets.14,109 Interestingly, for the first time, Tarn et al.111 revealed that sulfur oxidation/reduction and the utilization of methane and hydrogen were processes potentially important for sustaining the bottom hadal microbiome in the Mariana Trench. Subsequently, an investigation aiming to determine the functional capacities of hadal microbial populations was conducted in the Mariana Trench.112 Abundant hydrocarbon-degrading microorganisms affiliated within genera Oleibacter, Thalassolituus, and Alcanvivorax were observed to dominate the bottom seawater of the Challenger Deep. The metabolic prediction and transcription of genes found abundant putative genes closely associated with the metabolism of medium-chain, long-chain, and cyclic hydrocarbons. These findings were well aligned with the heterotrophic potentials revealed in previous studies.14,111 Several other studies, dedicated to elucidating the hadopelagic communities from the Japan, New Britain, and Kermadec Trenches, yielded similar results, finding that hadal microbial communities are significantly different from those above them.106,108,113
Although hadal bottom sediments remain largely unexplored, they have been shown to harbor high cell abundance, high metabolic activity, and large microbial communities. The special trench topography and frequent tectonic activities, such as seismicity and landslides, facilitate rapid accumulation of sinking and sedimentary organic matter,83,86,102,110 which largely contributes to the flourishing of prokaryotes in hadal sediments.15,104,114 Studies on the microbiology of hadal sediments began as early as the 1950s,104 and, in the last decade, with the application of omics analysis and deep-sea sampling techniques, knowledge of hadal sediment microbiomes and their potential functions related to geochemical cycles has greatly improved. A series of investigations—mainly concentrated on the west Pacific Ocean, including the Japan, Izu-Ogasawara, Mariana, Kermadec, Mussau, and Yap Trenches104,108,114,115—have recently been carried out to determine the microbial communities, adaptation mechanisms, and potential roles in hadal sediments. These studies suggest a significant distinction between abyssal plain and hadal trench sediments, and the dominant enrichment of heterotrophic bacterial populations, which are well correlated with organic matter degradation and recalcitrant material breakdown.15,108 These commonly dominant taxa are mainly derived from the bacterial Chloroflexi, Bacteroidetes, Planctomycetes, Marinimicrobia, and Gemmatimonadetes, and archaeal Woesearchaeota and Thaumarchaeota.104,115 These microbial lineages are inferred to participate in diverse geochemical cycles such as alkane degradation; the nitrogen cycle, including aerobic ammonium oxidization, nitrification, and denitrification; carbon assimilation; and chemoheterotrophic functions.15,104,108,114,115 Interestingly, the microbial cell abundance and community structures of hadal sediments usually show substantial differences among different subduction trenches.15,108,115 This is likely attributable to the influence of geographic separation, primary production in overlying surface seawaters, and the distinctions in tectonic activity and geochemical regimes.83
Hadal fauna
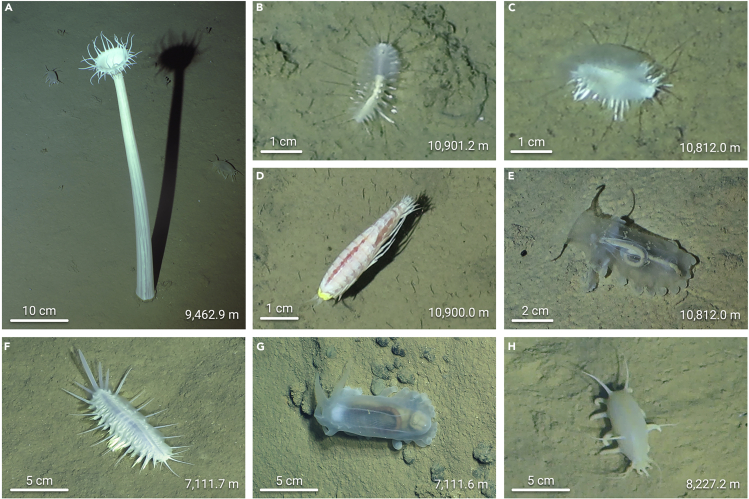
As a unique extreme environment, especially considering its ultra-high hydrostatic pressure, the hadal zone was once considered unsuitable for the survival of metazoans. However, in 1901, Echiuroidea, Asteroidea, Ophiuroidea, and benthic fish were successfully caught at a 6,035-m depth in the Moseley Trench during the Princess Alice expedition.116 In the following 100 years, with the rapid development of hadal exploration technology, many animal groups have been discovered in the hadal zone around the world, including crustaceans, echinoderms, mollusks, and many other large benthic animals. During the Fendouzhe cruise in 2020, novel species of hadal fauna were observed in the Challenge Deep region of the southern Mariana Trench (Figure 5). In some sites, the density of polychaetes and holothurians is high and reaches 2–4 individuals/m2, indicating a sufficient food supply for the benthic fauna in the deepest part of the world’s ocean.
Figure 5.
Novel species of hadal fauna observed by Fendouzhe at different water depths on the seafloor of the southern Mariana Trench
(A) Cnidarian.
(B) Polychaete.
(C) Polychaete.
(D) Amphipod.
(E) Holothurian.
(F) Polychaete.
(G) Holothurian.
(H) Holothurian.
Although we still do not fully understand what key environmental features are determining the abundance, composition, and diversity of the hadal community, the hadal environment affects organisms in many ways. However, the adaptation of hadal organisms to the environment is a comprehensive process involving morphological, physiological, and genetic aspects. Hadal animals show certain specificities in their adaptability to the hadal environment, but also some commonalities.
Morphological and behavior adaptation
In order to adapt to these extreme environments, hadal organisms have undergone adaptive evolution in terms of morphology and physiological functions. To adapt to high pressure, hadal liparids exhibit high volumes of gelatinous tissues, watery muscles, transparent skin and scales, thin and incompletely ossified bones, and a non-closed skull.117,118 The strong pharyngeal jaw apparatus and inflated stomachs found in hadal snailfishes have been speculated to increase their ability to feed on the large amphipod biomass.119,120
Although hadal invertebrates do not seem to be significantly different from shallow-living animals in morphology, there are more species showing strong mobility, which might help them to obtain the limited food in the hadal zone. For instance, deep-sea sea cucumbers from the families Pelagothuriidae, Laetmogonidae, Psychropotina, and Elpidiidae often show higher abilities to float and commonly swim faster.
Food utilization strategy
The hadal biosphere is dark, with almost no light. In contrast to the previous notion that scavengers only consume carrion, growing evidence suggests that the diets of hadal fauna are diverse and could also be derived from surface-derived particulate organic matter, chemosynthetic bacteria/invertebrates associated with seepage, invertebrates that scavenge amphipods, sediments, and phytoplankton.121,122 Although the sources of food in the hadal environment are relatively simple, the food in the hadal environment is not as scarce as was imagined. The most important source of nutrients in the trench is the organic matter deposits in the upper ocean.83 The bottom of the trench is composed of organic-rich sediments, which in turn nourish the proliferation of hadal bacteria, planktons, and animals, e.g., sea cucumbers, starfish, sea anemones, and mollusks. These chemoautotrophic and heterotrophic microorganisms may be among the most important food sources in the hadal ecosystem, directly or indirectly supplying benthic animals that feed on microorganisms and benthic organic debris.83,123
The carcasses of large higher animals such as fish and cetaceans in the upper ocean are high-quality foods that can quickly sink to the bottom, because they are rich in high-quality fatty acids and proteins. As a nutrient-rich food resource, this biological debris can quickly increase the biodiversity of a local area of the trench, thereby affecting the compositions of the hadal communities.123 Hadal amphipods are scavengers commonly found in the hadal zone, with strong mobility. Observations of the feeding behavior of hadal amphipods have found that such creatures can quickly locate and consume biological remains.16 Their perceptive and fast-swimming abilities may be adaptive evolution enabling feeding behavior appropriate for the unique food source of large hadal creature carcasses. However, how these benthic animals perceive biological debris and accurately position them in the vast hadal zone requires further research.
In some hadal trenches (such as the Puerto Rico, Kermadec, Palau, and Mariana Trenches), copious amounts of seaweed, sugarcane, coconut shell, and bamboo, and other terrestrial and coastal wetland plant debris, have been found.86,124, 125, 126, 127 A large number of organisms such as gastropods feed on terrestrial wood debris.123 Research on the digestive enzymes of the hadal amphipod Hirondellea gigas found that this type of organism has evolved a unique cellulase that can break down cellulose into glucose and cellobiose, and therefore is able to obtain nutrition from the deposited wood debris.128 These results indicate that the input of terrestrial organic matter may have promoted the development of these special animal groups in the hadal trench.
Molecular adaptation
The hadal zone is very harsh for most marine animals, but the hadal creatures that live in this extreme environment are well adapted to it. High hydrostatic pressure and low temperature can change the fluidity of biofilms, reduce the stability of protein structures, and inhibit enzyme activity,117,129 ultimately affecting physiological functions and activities. A low temperature will reduce enzyme activity and metabolic rates, thereby affecting the spatial distribution of hadal organisms.123 Hadal creatures have similar strategies for living in low-temperature environments such as that in the polar region, and can resist the severe, cold environment by the high expression of heat shock proteins and lipids. Research on the hadal amphipods found that gene families related to low-temperature adaptation exhibit either an enrichment contraction response or a positive selection effect in their adaptation to the low-temperature environment of the trench.129
Snailfish living in the hadal trench have high levels of trimethylamine N-oxide (TMAO).130 TMAO, which has an important physiological role and can stabilize protein structures, is synthetized by TMA oxidation in cells, which is catalyzed by the enzyme FAO. At around 8,200 m, the TMAO levels in hadal fish would reach the maximum tolerable; any further increase might cause osmoregulatory problems.130 The genome data of the hadal snailfish Pseudoliparis swirei show that its TMAO synthesis gene and heat shock protein hsp90 gene have undergone significant mutations.117 Analysis of the hadal amphipod transcriptome shows that its β-alanine gene, another important osmotic regulator, is subject to positive selection.129
Anthropogenic pollutants in hadal environments
Plastics and microplastics
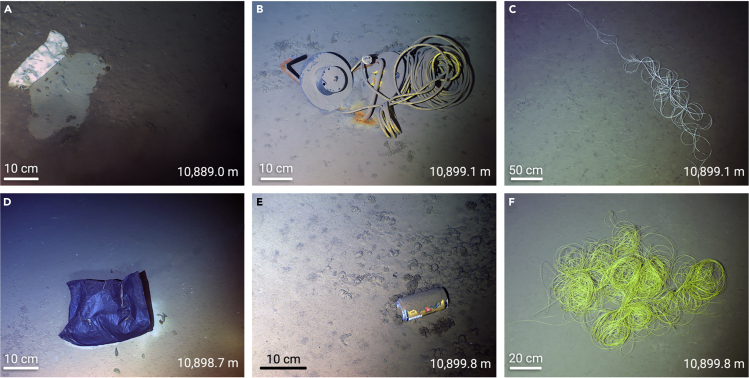
An estimated 4.8–12.7 Mt of plastics have entered the oceans131, of which only 0.26 Mt are floating in the seas, mostly in the form of microplastics (pieces less than 5 mm in diameter). The rest sinks and settles at the bottom as seafloor litter. Peng et al.132 documented the deep-sea debris in the submarine canyons of the northern South China Sea and found the highest density of marine debris (52,000 items per km2) at the seafloor, which is one order of magnitude higher than that reported in other locations worldwide. However, reports of plastics or other types of litter in hadal trenches >6,000 m are scarce. Chiba et al.107 analyzed the records of the Deep-sea Debris Database and revealed 3,425 litter items in the Pacific and Indian Oceans, of which 33% were plastics. The relative dominance of plastic debris was larger at depths greater than 6,000 m (52%) than at shallower depths (18%–22% at >1,000 m), and the debris was almost exclusively single-use plastic. The highest record is that of a plastic bag at 10,898 m in the Mariana Trench in the northwestern Pacific. Apart from plastics, there have also been reports of finding a raincoat in the Challenger Deep (the deepest point in the Mariana Trench),133 a beer can at 6,037 m in the Kermadec Trench and wine bottle at ∼8,228 m in the New Britain Trench during the DEEPSEA CHALLENGE Expedition,33 and a trash can at 7,216 m in the Ryuku Trench.134 During the Fendouzhe diving cruise in 2020, black and white plastic bags, an electric wire, a beer can, and fiber-optic tethers were observed in the Challenge Deep of the Mariana Trench (Figure 6). Those observations highlight the fact that plastics and other debris have contaminated the deepest part of the world’s ocean.
Figure 6.
Debris found on the seafloor of the Challenge Deep
(A) A fragment of white plastic bag.
(B) An electric wire.
(C) White fiber-optic tethers.
(D) A black plastic bag.
(E) A beer can.
(F)Yellow fiber-optic tethers.
Microplastics, defined as plastic fragments ranging in size between 0.1 μm and 5 mm, have been recorded in various studies in coastal e.g.135 and deep-sea sediments e.g.136,137 to hadal trenches.35,138,139 The adverse effects of microplastic ingestion, although not fully understood, are known to affect about 700 marine species.140 Peng et al.35 first documented the presence of microplastics in the bottom water and sediments of the southern Mariana Trench, at depths ranging from 2,500 to 11,000 m. The microplastic abundances in the bottom waters ranged from 2.06 to 13.51 pieces/L and became more concentrated with depth. At 10,903 m, 11.43 pieces/L were detected in the trench water samples, four times higher than that reported in the subsurface water of open seas, including the NE Pacific Ocean,141 North Pacific Gyre,142 South Pacific subtropical gyre,143 North Atlantic Ocean,144 and Arctic Ocean.145,146 The hadal sediments of the Mariana Trench were also abundant in microplastics, ranging from 200 to 2,200 pieces/L of dry weight sediment, with a maximum of 2,200 pieces/L at the depth of 7,180 m, followed by 2,000 pieces/L at 9373 m. Compared with worldwide data, the microplastic abundances in the Mariana sediments are twice as high as those reported in deep-sea sediments from the Atlantic Ocean and Mediterranean Sea (70–800 pieces/L).147 Microplastics were also reported to be present in the Kuril Kamchatka Trench at depths of 5,143–8,250 m, with concentrations of 14–209 pieces/kg of sediment (dry weight). The specific sources of these microplastics are largely unknown, but could be multiple, including land-based, heavily industrialized areas148 or oceanic sources, such as the so-called Great Pacific Garbage Patch.149
It is now evident that microplastics have invaded the deepest trenches on Earth and consequently pose high risks to the benthic fauna in the deep oceans. Jamieson et al.139 examined the extent of microplastic pollution affecting lysianassoid amphipods across multiple hadal trenches around the Pacific Rim, including the Peru-Chile Trench in the southeast Pacific; the New Hebrides and Kermadec Trenches in the southwest Pacific; and the Japan, Izu-Bonin, and Mariana Trenches in the Northwest Pacific. The sampling depths ranged from 7,000 to 10,890 m, the deepest point being the Challenger Deep. They found microfibers in the hindguts of amphipods at all the nine sites they sampled. Interestingly, the highest frequency of ingestion occurred in the amphipods from the Mariana Trench (10,890 m). In spite of the fact that the six trenches are bathymetrically and geographically isolated by large distances,139 microplastics are ubiquitous in the hadal trenches and bioavailable to the hadal amphipods studied to date. Moreover, the presence of microplastics in the guts of hadal amphipods could result in a perpetual trophic transfer within the hadal communities, where the amphipods, on one hand, serve as prey to higher species and, on the other, ingest microplastics from carrion falls containing the remains of the predators through the water column.139 A study on penetration of bomb 14C into hadal trenches also points out that the anthropogenic pollution can reach the deepest ocean trench rapidly via the food chain.150 It becomes more apparent that the funneling effect associated with the V-shaped trench morphology,14,35 accompanied by occasional earthquakes, the repeated resuspension of materials,100 and bottom currents, plays a vital role in the accumulation of microplastics in the trenches. A relatively rapid deposition of sediments has also been reported in the hadal zone of the Mariana Trench.27 The hadal trenches act as a sink for microplastics in the ocean and account for a part of the “missing” plastics. The extent of proliferation, though, will not be limited to the hadal zone. Microplastics will transfer within food webs and be bioavailable to species inhabiting shallower depths. Further studies are needed to monitor the abundance of microplastics within the broader hadal environment and understand their impact on marine fauna.
POPs
Despite being banned by most countries since the late 1970s,151 POPs have been the focus of extensive studies in the past few decades. Compounds such as polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) have been detected in both terrestrial152 and marine environments.153, 154, 155, 156 However, POPs in hadal trenches were first explored by Jamieson et al.,148 who reported high concentrations of POPs in endemic amphipod fauna from the Mariana and Kermadec Trenches, at depths of 7,000–10,000 m. This was a pioneering find that resulted in a series of further attempts by scientists to explore hadal trench pollution. Jamieson et al.148 used deep-sea landers and baited traps to capture three species of amphipods: Hirondellea dubia and Bathycallisoma schellenbergi (from the Kermadec Trench), and H. gigas (from the Mariana Trench). The concentrations of PCBs and PBDEs in these crustaceans were higher than baseline levels and 50 times greater than in crabs from a highly polluted river system in China. The high PCB concentrations in the Mariana Trench could be due to its proximity to highly industrialized areas and the Great Pacific Garbage Patch. In a more recent study, elevated levels of PCBs, PBDEs, and chlorinated pesticides such as DDT (dichlorodiphenyltrichloroethane) isomers were detected in hadal amphipods from the Mariana, Mussau, and New Britain Trenches.156 The high amounts of POPs in these amphipods could be due to bioaccumulation or their feeding mechanisms, where they ingest contaminated particles associated with particulate matter or carrion falls sinking from the surface to the deep waters through the water column.148,156 Besides amphipods, PCBs and PBDEs were also detected in the Mariana Trench sediments.157 The overall ΣPCB values from their study were overwhelmingly higher than those from the shallow marine sediments studied in the past, and higher than the world baseline levels for ΣPCBs arising from atmospheric transport found in clean coastal sediments, which are <1 ng/g dry weight.158 However, the PCB concentrations in the sediments are markedly lower than those found in the endemic amphipods by Jamieson et al.148, possibly because, unlike in sediments, the concentrations of contaminants in these amphipods are multiplied over time by bioaccumulation. Apart from the notion that POPs are bound by organic matter, Dasgupta et al.157 argued that clay minerals may play an important role in the adsorption of these particles in hadal sediments. This is supported by the fact that a large number of clay minerals were detected in their study, and such adsorption mechanisms (e.g., Li et al. 159) likely agglomerate the POPs in the deepest trench sediments.
Hg and other toxic heavy elements
Hg in the form of monomethylmercury (CH3Hg+) readily bioaccumulates and is thought to be the primary toxic form entering the human body.160 However, the pathways of Hg accumulation in hadal trenches are little understood. Sun et al.161 measured the total Hg (THg) and methyl Hg (MeHg) concentrations and Hg isotopic compositions of amphipods from the Mariana and nearby Yap Trench, as well as one snailfish from the Yap Trench. The THg concentrations were comparable with those of their counterparts from the abyssal Arctic Ocean, but higher than those of benthic organisms in coastal and freshwater ecosystems. They argued that the isotopic composition of THg in each sample was representative of the isotopic composition of MeHg in that sample and that deep ocean MeHg is supplied from the upper ocean by particles sinking rather than by in situ microbial methylation. This argument is further supported by Blum et al.,162 who suggested that anthropogenic Hg in the marine food web at∼500 m is transported to deep-sea trenches primarily through carrion in the water column, and integrated within the endemic fauna in hadal trenches. Liu et al.34 measured the THg and MeHg in amphipods from the Mariana, Massau, and New Britain Trenches, at depths of 6,990 to 10,840 m. They found that THg and MeHg concentrations were higher in trench amphipods than in amphipods from coastal and freshwater environments and suggested that Hg bioaccumulation in the hadal trenches depends on food availability, which is closely related to the surface ocean’s biogeochemical characteristics, such as lateral transport, methylation, and MeHg bioaccumulation in food webs. Fatty acid analysis of samples confirmed that these hadal species were feeding on carrion from higher-trophic-level predators.
A few studies have also reported the presence of heavy toxic elements (such as, Cd, Co, Pb, and Cu) in deep hadal trenches, primarily because these elements can negatively affect marine species when ingested and transferred to humans, potentially causing organ failure.163 Sediment samples from the Yap Trench were analyzed for heavy metals164; the study revealed that the enrichment of heavy metals in the area was influenced by natural factors and not anthropogenic sources. The patterns of heavy-element concentration in snailfish from the Mariana and Kermadec Trenches were also assessed in a separate study by Welty et al.165 Specifically, they determined the concentrations of As, Cd, Ba, Ni, Cu, Co, Pb, Se, Cr, and Hg in five different species of fish ranging from 1,000 m to 7,626 m. Unexpectedly, Ni and Cr were highly abundant in the Kermadec snailfish, while Cd and Pb showed the highest values in the Mariana samples. Their study, however, did not discuss the origins or sources of these pollutants.
Conclusions and future direction
Recent investigations have shed light on the unique geological, environmental, and biological processes that take place on, beneath, and above the seafloor of the hadal trench. However, as the most inaccessible ocean environment on Earth, the hadal trench still remains largely enigmatic, and our knowledge about the hadal trench is just the “tip of the iceberg.” To date, only a few hadal trenches have been investigated sporadically and a large portion of hadal trenches around the world remain untouched. Like tropical rain forest systems and deep-subsurface biospheres, hadal trench systems remain among the least investigated and most mysterious habitats on Earth, making them one of the last frontiers on our planet.
Compared with what we know about hadal ecology, our knowledge about hadal geology and the hadal environment, as well as the interactions among geological, environmental, and biological processes in the hadal trench, is very limited. Future directions of hadal research include (1) biogeographic distribution of the hadal fauna and gene flow; (2) origin, evolution, and environmental adaptation of the hadal fauna; (3) response of the hadal ecosystem to the upper ocean and global climate change; (4) distribution and energy source of the hadal subsurface biosphere; (5) tectonic and petrologic heterogeneity of hadal trenches; (6) geochemical effects of hydration of hadal lithosphere; (7) fluid activities and associated chemosynthetic communities at hadal depths; (8) biogeochemical cycles and diagenetic pathways in hadal sediments; (and 9) ecological and toxic effect of anthropogenic pollutants on hadal fauna.
Currently, technologies that can be used in the exploration of hadal environment are highly limited. Immovable instruments such as landers with weak operation capacity have long served as major equipment for the investigation of hadal environments, although they have been demonstrated to be economic and effective methods for such investigation. Only through the dedicated development of hadal technologies, especially manned submersibles, remotely operated vehicles, automated underwater vehicles, chemical and physical sensors, and in situ experimental instruments that would revolutionize hadal exploration, will we be able to fully understand the intrinsic links between the geological, geochemical, environmental, and biological processes in the hadal trench. With the increased application and maturity of full-ocean-depth submersibles, such as Fendouzhe and Limiting Factor, the second wave of hadal exploration in the world is expected to be imminent. During the upcoming hadal exploration campaign, international cooperation through sharing cruises, samples, and data will be significant for enabling us to understand the deepest regions of the Earth’s oceans. The knowledge from hadal trenches, together with that from shallower ocean, will help us better comprehend the Earth’s comprehensive marine system.
Acknowledgments
We are indebted to the sea-trial team and the pilots of the manned submersible Fendouzhe for their professional operation. We also thank Fulin Zeng from Shangdong University for her creation of the graphic abstract. This work was financially supported by the National Key Research and Development Program of China (grant nos. 2016YFC0300503, 2016YFC0300600, 2016YFC0304900).
Author contributions
X.P. designed and organized this manuscript. M.D., X.P., H.Z., S.D., J.L., J.L., and S.L. contributed to the writing of this manuscript. H.X., C.C., H.J., H.X., J.L., S.H., L.H., S. Cai, S. Chen, and K.T. gave suggestions of conceptual ideas for this manuscript. C.Y. provided high-definition images taken by manned submersible Fendouzhe. All authors discussed, revised, and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published Online: April 30, 2021
Lead contact website
References
- 1.Jamieson A.J., Fujii T., Mayor D.J., et al. Hadal trenches: the ecology of the deepest places on Earth. Trend Ecol. Evol. 2010;25:190–197. doi: 10.1016/j.tree.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Stern R.J. In: Encyclopedia of Geology. Second Edition. Alderton D., Elias andS.A., editors. Academic Press; 2021. Ocean Trenches; pp. 845–854. [Google Scholar]
- 3.Stern R.J. Subduction zones. Rev. Geophys. 2002;40:3-1–3-38. [Google Scholar]
- 4.Fryer P., Wheat C.G., Mottl M.J. Mariana blueschist mud volcanism: Implications for conditions within the subduction zone. Geology. 1999;27:103. [Google Scholar]
- 5.Grellet C., Dubois J. The depth of trenches as a function of the subduction rate and age of the lithosphere. Tectonophysics. 1982;82:45–56. [Google Scholar]
- 6.Bry M., White N. Reappraising elastic thickness variation at oceanic trenches. J. Geophys. Res. Solid Earth. 2007;112:B08414. [Google Scholar]
- 7.Stewart H.A., Jamieson A.J. Habitat heterogeneity of hadal trenches: Considerations and implications for future studies. Prog. Oceanogr. 2018;161:47–65. [Google Scholar]
- 8.Wolff T. The hadal community, an introduction. Deep-Sea Res. 1959;6:95–124. [Google Scholar]
- 9.Spärck, R. (1952). Revealing the secrets of the deep. The Galathea Expedition of 1950-52. Dan For Off J. 6, 1-6.
- 10.Jamieson A.J., Fujii T., Solan M., et al. HADEEP: free-falling landers to the deepest places on Earth. Mar. Technol. Soc. J. 2009;43:151–160. [Google Scholar]
- 11.ZoBell C.E. Bacterial life at the bottom of the Philippine Trench. Science. 1952;115:507–508. doi: 10.1126/science.115.2993.507. [DOI] [PubMed] [Google Scholar]
- 12.Yayanos A.A. Microbiology to 10,500 meters in the deep sea. Annu. Rev. Microbiol. 1995;49:777–805. doi: 10.1146/annurev.mi.49.100195.004021. [DOI] [PubMed] [Google Scholar]
- 13.Tamburini C., Boutrif M., Garel M., et al. Prokaryotic responses to hydrostatic pressure in the ocean--a review. Environ. Microbiol. 2013;15:1262–1274. doi: 10.1111/1462-2920.12084. [DOI] [PubMed] [Google Scholar]
- 14.Nunoura T., Takaki Y., Hirai M., et al. Hadal biosphere: Insight into the microbial ecosystem in the deepest ocean on Earth. Proc. Natl. Acad. Sci. U S A. 2015;112:E1230–E1236. doi: 10.1073/pnas.1421816112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiraoka S., Hirai M., Matsui Y., et al. Microbial community and geochemical analyses of trans-trench sediments for understanding the roles of hadal environments. ISME J. 2019;14:1–17. doi: 10.1038/s41396-019-0564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamieson A.J., Fujii T., Solan M., et al. Liparid and macrourid fishes of the hadal zone: in situ observations of activity and feeding behaviour. Proc. Biol. Sci. 2009;276:1037–1045. doi: 10.1098/rspb.2008.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todo Y., Kitazato H., Hashimoto J., et al. Simple Foraminifera flourish at the ocean's deepest point. Science. 2005;307:689. doi: 10.1126/science.1105407. [DOI] [PubMed] [Google Scholar]
- 18.Wolff T. The concept of the hadal or ultra-abyssal fauna. Deep Sea Research and Oceanographic Abstracts. 1970;17:983–1003. [Google Scholar]
- 19.Angel M.V. Ocean trench conservation. Environmentalist. 1982;2:1–17. [Google Scholar]
- 20.Liu R., Wang L., Wei Y., et al. The hadal biosphere: Recent insights and new directions. Deep Sea Res. Part II. 2018;155:11–18. [Google Scholar]
- 21.Fujioka K., Okino K., Kanamatsu T., et al. Morphology and origin of the Challenger Deep in the southern Mariana Trench. Geophys. Res. Lett. 2002;29:1372. [Google Scholar]
- 22.Ranero C.R., Phipps Morgan J., McIntosh K., et al. Bending-related faulting and mantle serpentinization at the Middle America trench. Nature. 2003;425:367–373. doi: 10.1038/nature01961. [DOI] [PubMed] [Google Scholar]
- 23.Grevemeyer I., Kaul N., Diaz-Naveas J.L., et al. Heat flow and bending-related faulting at subduction trenches: Case studies offshore of Nicaragua and central Chile. Earth Planet. Sci. Lett. 2005;236:238–248. [Google Scholar]
- 24.Taira K., Yanagimoto D., Kitagawa S. Deep CTD casts in the Challenger Deep, Mariana Trench. J. Oceanogr. 2005;61:447–454. [Google Scholar]
- 25.Kawagucci S., Makabe A., Kodama T., et al. Hadal water biogeochemistry over the Izu–Ogasawara Trench observed with a full-depth CTD-CMS. Ocean Sci. 2018;14:575–588. [Google Scholar]
- 26.Danovaro R., Della Croce N., Dell’Anno A., et al. A depocenter of organic matter at 7800m depth in the SE Pacific Ocean. Deep Sea Res. Part I. 2003;50:1411–1420. [Google Scholar]
- 27.Glud R.N., Wenzhöfer F., Middelboe M., et al. High rates of microbial carbon turnover in sediments in the deepest oceanic trench on Earth. Nat. Geosci. 2013;6:284–288. [Google Scholar]
- 28.Leduc D., Rowden A.A. Nematode communities in sediments of the Kermadec Trench, southwest Pacific Ocean. Deep Sea Res. Part I. 2018;134:23–31. [Google Scholar]
- 29.Luo M., Glud R.N., Pan B., et al. Benthic carbon mineralization in hadal trenches: insights from in situ determination of benthic oxygen consumption. Geophys. Res. Lett. 2018;45:2752–2760. [Google Scholar]
- 30.Luo M., Gieskes J., Chen L., et al. Sources, degradation, and transport of organic matter in the New Britain Shelf-Trench Continuum, Papua New Guinea. J. Geophys. Res. Biogeosci. 2019;124:1680–1695. [Google Scholar]
- 31.Glud, R., Thamdrup, B., Zabel, M., et al. (2020) Deposition and early diagenesis of organic material in hadal trenches. In Ocean Sciences Meeting 2020. AGU.
- 32.Thamdrup, B., Schauberger, C., Larsen, M., et al. (2020) Benthic nitrogen cycling in hadal trenches: High rates and large contributions from anammox. In Ocean Sciences Meeting 2020. AGU.
- 33.Jamieson A.J. A contemporary perspective on hadal science. Deep Sea Res. Part II. 2018;155:4–10. [Google Scholar]
- 34.Liu M., Xiao W., Zhang Q., et al. Methylmercury bioaccumulation in deepest ocean fauna: implications for ocean mercury biotransport through food webs. Environ. Sci. Technol. Lett. 2020;7:469–476. [Google Scholar]
- 35.Peng X., Chen M., Chen S., et al. Microplastics contaminate the deepest part of the world’s ocean. Geochem. Perspect. Lett. 2018;9:1–5. [Google Scholar]
- 36.Peacock S.M. Are the lower planes of double seismic zones caused by serpentine dehydration in subducting oceanic mantle? Geology. 2001;29:299–302. [Google Scholar]
- 37.Faccenda M., Gerya T., Burlini L. Deep slab hydration induced by bending-related variations in tectonic pressure. Nat. Geosci. 2009;2:790–793. [Google Scholar]
- 38.Ranero R.C., Sallares V. Geophysical evidence for hydration of the crust and mantle of the Nazca plate during bending at the north Chile Trench. Geology. 2004;32:549–552. [Google Scholar]
- 39.Grevemeyer I., Ranero C.R., Flueh E.R., et al. Passive and active seismological study of bending-related faulting and mantle serpentinization at the Middle America trench. Earth Planet. Sci. Lett. 2007;258:528–542. [Google Scholar]
- 40.Naif S., Key K., Constable S., et al. Water-rich bending faults at the Middle America Trench. Geochem. Geophys. Geosyst. 2015;16:2582–2597. [Google Scholar]
- 41.Contreras-Reyes E., Grevemeyer I., Flueh E.R., et al. Upper lithospheric structure of the subduction zone offshore of southern Arauco Peninsula, Chile, at ∼38°S. J. Geophys. Res. Solid Earth. 2008;113:B07303. [Google Scholar]
- 42.Wan K., Lin J., Xia S., et al. Deep seismic structure across the southernmost Mariana Trench: implications for arc rifting and plate hydration. J. Geophys. Res. Solid Earth. 2019;124:4710–4727. [Google Scholar]
- 43.Eimer M., Wiens D.A., Cai C., et al. Seismicity of the incoming plate and forearc near the Mariana Trench recorded by ocean bottom seismographs. Geochem. Geophys. Geosyst. 2020;21 e2020GC008953. [Google Scholar]
- 44.Van Avendonk H.J.A., Holbrook W.S., Lizarralde D., et al. Structure and serpentinization of the subducting Cocos plate offshore Nicaragua and Costa Rica. Geochem. Geophys. Geosyst. 2011;12:1–23. [Google Scholar]
- 45.Lefeldt M., Ranero C.R., Grevemeyer I. Seismic evidence of tectonic control on the depth of water influx into incoming oceanic plates at subduction trenches. Geochem. Geophys. Geosyst. 2012;13:1–17. [Google Scholar]
- 46.Bostock M.G., Hyndman R.D., Rondenay S., et al. An inverted continental Moho and serpentinization of the forearc mantle. Nature. 2002;417:536–538. doi: 10.1038/417536a. [DOI] [PubMed] [Google Scholar]
- 47.Reynard B. Serpentine in active subduction zones. Lithos. 2013;178:171–185. [Google Scholar]
- 48.Kamimura A., Kasahara J., Shinohara M., et al. Crustal structure study at the Izu-Bonin subduction zone around 31°N: implications of serpentinized materials along the subduction plate boundary. Phys. Earth Planet. Inter. 2002;132:105–129. [Google Scholar]
- 49.Tibi R., Wiens D.A., Yuan X. Seismic evidence for widespread serpentinized forearc mantle along the Mariana convergence margin. Geophys. Res. Lett. 2008;35:L13303. [Google Scholar]
- 50.Kirby S., Engdahl R.E., Denlinger R. In: Bebout G.E., Scholl D.W., Kirby S.H., Platt J.P., editors. Vol. 96. American Geophysical Union); 1996. Intermediate-depth intraslab earthquakes and arc volcanism as physical expressions of crustal and uppermost mantle metamorphism in subducting slabs; pp. 195–214. (Subduction: top to bottom). [Google Scholar]
- 51.Kerrick D.M., Connolly J. Metamorphic devolatilization of subducted marine sediments and the transport of volatiles into the Earth's mantle. Nature. 2001;411:293–296. doi: 10.1038/35077056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kerrick D.M., Connolly J.A.D. Metamorphic devolatilization of subducted oceanic metabasalts: implications for seismicity, arc magmatism and volatile recycling. Earth Planet. Sci. Lett. 2001;189:19–29. [Google Scholar]
- 53.Moore J., Saffer D.M. Updip limit of the seismogenic zone beneath the accretionary prism of southwest Japan: An effect of diagenetic to low-grade metamorphic processes and increasing effective stress. Geology. 2001;29:183. [Google Scholar]
- 54.Fryer P., Gharib J., Ross K., et al. Variability in serpentinite mudflow mechanisms and sources: ODP drilling results on Mariana forearc seamounts. Geochem. Geophys. Geosyst. 2006;7:1–15. [Google Scholar]
- 55.Fryer P. Serpentinite mud volcanism: Observations, processes, and implications. Annu. Rev. Mar. Sci. 2012;4:345–373. doi: 10.1146/annurev-marine-120710-100922. [DOI] [PubMed] [Google Scholar]
- 56.Kerrick D. Serpentinite seduction. Science. 2002;298:1344–1345. doi: 10.1126/science.298.5597.1344. [DOI] [PubMed] [Google Scholar]
- 57.Ohara Y., Reagan M.K., Fujikura K., et al. A serpentinite-hosted ecosystem in the southern Mariana forearc. Proc. Natl. Acad. Sci. U S A. 2012;109:2831–2835. doi: 10.1073/pnas.1112005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogawa Y., Fujioka K., Fujikura K., et al. En echelon patterns of Calyptogena colonies in the Japan Trench. Geology. 1996;24:807. [Google Scholar]
- 59.Du M., Peng X., Seyfried W.E., et al. Fluid discharge linked to bending of the incoming plate at the Mariana subduction zone. Geochem. Perspect. Lett. 2019;11:1–5. [Google Scholar]
- 60.Peng X., Guo Z., Du M., et al. Past endolithic life in metamorphic ocean crust. Geochem. Perspect. Lett. 2020;14:14–19. [Google Scholar]
- 61.Oguri K., Kawamura K., Sakaguchi A., et al. Hadal disturbance in the Japan Trench induced by the 2011 Tohoku–Oki earthquake. Sci. Rep. 2013;3:1915. doi: 10.1038/srep01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McHugh C., Kanamatsu T., Seeber L., et al. Remobilization of surficial sediment triggered by the A.D. 2011 Mw9 Tohoku-Oki earthquake and tsunami along the Japan Trench. Geology. 2016;44:391. [Google Scholar]
- 63.Evans K.A. The redox budget of subduction zones. Earth Sci. Rev. 2012;113:11–32. [Google Scholar]
- 64.Kelemen P.B., Manning C.E. Reevaluating carbon fluxes in subduction zones, what goes down, mostly comes up. Proc. Natl. Acad. Sci. U S A. 2015;112:E3997–E4006. doi: 10.1073/pnas.1507889112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelley D., Karson J., Blackman D., et al. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30 degrees N. Nature. 2001;412:145–149. doi: 10.1038/35084000. [DOI] [PubMed] [Google Scholar]
- 66.Kelley D.S., Karson J.A., Früh-Green G.L., et al. A serpentinite-hosted ecosystem: The Lost City hydrothermal field. Science. 2005;307:1428–1434. doi: 10.1126/science.1102556. [DOI] [PubMed] [Google Scholar]
- 67.Michibayashi K., Ohara Y., Stern R.J., et al. Peridotites from a ductile shear zone within back-arc lithospheric mantle, southern Mariana Trench: Results of a Shinkai 6500 dive. Geochem. Geophys. Geosyst. 2009;10:Q05X06. [Google Scholar]
- 68.Reagan M.K., McClelland W.C., Girard G., et al. The geology of the southern Mariana fore-arc crust: Implications for the scale of Eocene volcanism in the western Pacific. Earth Planet. Sci. Lett. 2013;380:41–51. [Google Scholar]
- 69.Nan J., King H., Delen G., et al. The nanogeochemistry of abiotic carbonaceous matter in serpentinites from the Yap Trench, western Pacific Ocean. Geology. 2020;49:330–334. [Google Scholar]
- 70.Onishi Y., Yamanaka T., Okumura T., et al. Evaluation of nutrient and energy sources of the deepest known serpentinite-hosted ecosystem using stable carbon, nitrogen, and sulfur isotopes. PLoS One. 2018;13:e0199000. doi: 10.1371/journal.pone.0199000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hand K.P., Bartlett D.H., Fryer P., et al. Discovery of novel structures at 10.7 km depth in the Mariana Trench may reveal chemolithoautotrophic microbial communities. Deep Sea Res. Part I. 2020;160:103238. [Google Scholar]
- 72.Edwards A.B. The formation of iddingsite. Am. Mineral. 1938;23:277–281. [Google Scholar]
- 73.Kuebler K.E. A comparison of the iddingsite alteration products in two terrestrial basalts and the Allan Hills 77005 Martian meteorite using Raman spectroscopy and electron microprobe analyses. J. Geophys. Res. Planets. 2013;118:803–830. [Google Scholar]
- 74.Stevens T.O., McKinley J.P. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science. 1995;270:450–455. [Google Scholar]
- 75.Mayhew L., Ellison E., McCollom T., et al. Hydrogen generation from low-temperature water-rock reactions. Nat. Geosci. 2013;6:478–484. [Google Scholar]
- 76.Torsvik T., Furnes H., Muehlenbachs K., et al. Evidence for microbial activity at the glass-alteration interface in oceanic basalts. Earth Planet. Sci. Lett. 1998;162:165–176. [Google Scholar]
- 77.Furnes H., Staudigel H. Biological mediation in ocean crust alteration: how deep is the deep biosphere? Earth Planet. Sci. Lett. 1999;166:97–103. [Google Scholar]
- 78.Furnes H., McLoughlin N., Muehlenbachs K., et al. In: Links Between Geological Processes, Microbial Activities and Evolution of Life. Dilek Y., Furnes H., Muehlenbachs andK., editors. Springer; 2008. Oceanic pillow lavas and hyaloclastites as habitats for microbial life through time - a review; pp. 1–68. [Google Scholar]
- 79.Plümper O., King H.E. Subduction zone forearc serpentinites as incubators for deep microbial life. Proc. Natl. Acad. Sci. U S A. 2017;114:4324–4329. doi: 10.1073/pnas.1612147114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suess E. In: Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate. Wilkes H., editor. Springer; 2018. Marine cold seeps: background and recent advances; pp. 1–21. [Google Scholar]
- 81.Yoshihiro F., Chiaki K., Noriaki M., et al. Dual symbiosis in the cold-seep thyasirid clam Maorithyas hadalis from the hadal zone in the Japan Trench, western Pacific. Mar. Ecol. Prog. Ser. 2001;214:151–159. [Google Scholar]
- 82.Eloe E.A., Shulse C.N., Fadrosh D.W., et al. Compositional differences in particle-associated and free-living microbial assemblages from an extreme deep-ocean environment. Environ. Microbiol. Rep. 2011;3:449–458. doi: 10.1111/j.1758-2229.2010.00223.x. [DOI] [PubMed] [Google Scholar]
- 83.Jamieson A. Cambridge University Press; 2015. The Hadal Zone: Life in the Deepest Oceans. [Google Scholar]
- 84.Ishiwatari R., Yamada K., Matsumoto K., et al. In: Dynamics and Characterization of Marine Organic Matter. Handa N., Tanoue E., Hama T., editors. Kluwer); 2000. Source of organic matter in sinking particles in the Japan Trench: molecular composition and carbon isotopic analyses; pp. 141–168. [Google Scholar]
- 85.Li D., Zhao J., Yao P., et al. Spatial heterogeneity of organic carbon cycling in sediments of the northern Yap Trench: Implications for organic carbon burial. Mar. Chem. 2020;223:103813. [Google Scholar]
- 86.Luo M., Gieskes J., Chen L., et al. Provenances, distribution, and accumulation of organic matter in the southern Mariana Trench rim and slope: Implication for carbon cycle and burial in hadal trenches. Mar. Geol. 2017;386:98–106. [Google Scholar]
- 87.Gallo N.D., James C., Kevin H., et al. Submersible- and lander-observed community patterns in the Mariana and New Britain trenches: Influence of productivity and depth on epibenthic and scavenging communities. Deep Sea Res. Part I. 2015;99:119–133. [Google Scholar]
- 88.Katsunori F., Shigeaki K., Kensaku T., et al. The deepest chemosynthesis-based community yet discovered from the hadal zone, 7326 m deep, in the Japan Trench. Mar. Ecol. Prog. Ser. 1999;190:17–26. [Google Scholar]
- 89.Stern R.J., Kohut E., Bloomer S.H., et al. Subduction factory processes beneath the Guguan cross-chain, Mariana Arc: no role for sediments, are serpentinites important? Contrib. Mineral. Petrol. 2006;151:202–221. [Google Scholar]
- 90.Chen Z., Li J., Li X., et al. Characteristics and implications of isoprenoid and hydroxy tetraether lipids in hadal sediments of Mariana and Yap Trenches. Chem. Geol. 2020;551:119742. [Google Scholar]
- 91.Xiao W., Wang Y., Liu Y., et al. Predominance of hexamethylated 6-methyl branched glycerol dialkyl glycerol tetraethers in the Mariana Trench: source and environmental implication. Biogeosciences. 2020;17:2135–2148. [Google Scholar]
- 92.Xu Y., Jia Z., Xiao W., et al. Glycerol dialkyl glycerol tetraethers in surface sediments from three Pacific trenches: Distribution, source and environmental implications. Org. Geochem. 2020;147:104079. [Google Scholar]
- 93.Ta K., Peng X., Xu H., et al. Distributions and sources of glycerol dialkyl glycerol tetraethers in sediment cores from the Mariana subduction zone. J. Geophys. Res. Biogeosci. 2019;124:857–869. [Google Scholar]
- 94.Harry W., Green I., Houston H. The mechanics of deep earthquakes. Annu. Rev. Earth Planet. Sci. 1995;23:169–213. [Google Scholar]
- 95.Dobson D.P., Meredith P.G., Boon S.A. Simulation of subduction zone seismicity by dehydration of serpentine. Science. 2002;298:1407–1410. doi: 10.1126/science.1075390. [DOI] [PubMed] [Google Scholar]
- 96.Kioka A., Schwestermann T., Moernaut J., et al. Event stratigraphy in a hadal oceanic trench: The Japan Trench as sedimentary archive recording recurrent giant subduction zone earthquakes and their role in organic carbon export to the deep sea. Front. Earth. Sci. 2019;7:319. [Google Scholar]
- 97.Bao R., Strasser M. Tectonically-triggered sediment and carbon export to the hadal zone. Nat. Commun. 2018;9:121. doi: 10.1038/s41467-017-02504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kioka A., Schwestermann T., Moernaut J., et al. Megathrust earthquake drives drastic organic carbon supply to the hadal trench. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-38834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Longhurst A., Sathyendranath S., Platt T., et al. An estimate of global primary production in the ocean from satellite radiometer data. J. Plankton Res. 1995;17:1245–1271. [Google Scholar]
- 100.Itou M., Matsumura I., Noriki S. A large flux of particulate matter in the deep Japan Trench observed just after the 1994 Sanriku-Oki earthquake. Deep Sea Res. Part I. 2000;47:1987–1998. [Google Scholar]
- 101.Turnewitsch R., Falahat S., Stehlikova J., et al. Recent sediment dynamics in hadal trenches: Evidence for the influence of higher-frequency (tidal, near-inertial) fluid dynamics. Deep Sea Res. Part I. 2014;90:125–138. [Google Scholar]
- 102.Wenzhöfer F., Oguri K., Middelboe M., et al. Benthic carbon mineralization in hadal trenches: Assessment by in situ O2 microprofile measurements. Deep Sea Res. Part I. 2016;116:276–286. [Google Scholar]
- 103.Nunoura T., Nishizawa M., Kikuchi T., et al. Molecular biological and isotopic biogeochemical prognoses of the nitrification-driven dynamic microbial nitrogen cycle in hadopelagic sediments. Environ. Microbiol. 2013;15:3087–3107. doi: 10.1111/1462-2920.12152. [DOI] [PubMed] [Google Scholar]
- 104.Nunoura T., Nishizawa M., Hirai M., et al. Microbial diversity in sediments from the bottom of the Challenger Deep, the Mariana Trench. Microbes Environ. 2018;33:186–194. doi: 10.1264/jsme2.ME17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu S., Peng X. Organic matter diagenesis in hadal setting: Insights from the pore-water geochemistry of the Mariana Trench sediments. Deep Sea Res. Part I. 2019;147:22–31. [Google Scholar]
- 106.Nunoura T., Hirai M., Takashima Y., et al. Distribution and niche separation of planktonic microbial communities in the water columns from the surface to the hadal waters of the Japan Trench under the eutrophic ocean. Front. Microbiol. 2016;7:1261. doi: 10.3389/fmicb.2016.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiba S., Saito H., Fletcher R., et al. Human footprint in the abyss: 30 year records of deep-sea plastic debris. Marine Policy. 2018;96:204–212. [Google Scholar]
- 108.Peoples L.M., Grammatopoulou E., Pombrol M., et al. Microbial community diversity within sediments from two geographically separated hadal trenches. Front. Microbiol. 2019;10:347. doi: 10.3389/fmicb.2019.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eloe E.A., Fadrosh D.W., Novotny M., et al. Going deeper: metagenome of a hadopelagic microbial community. PLoS One. 2011;6:e20388. doi: 10.1371/journal.pone.0020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ichino M.C., Clark M.R., Drazen J.C., et al. The distribution of benthic biomass in hadal trenches: A modelling approach to investigate the effect of vertical and lateral organic matter transport to the seafloor. Deep Sea Res. Part I. 2015;100:21–33. [Google Scholar]
- 111.Tarn J., Peoples L., Hardy K., et al. Identification of free-living and particle-associated microbial communities present in hadal regions of the Mariana Trench. Front. Microbiol. 2016;7:665. doi: 10.3389/fmicb.2016.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu J., Zheng Y., Lin H., et al. Proliferation of hydrocarbon-degrading microbes at the bottom of the Mariana Trench. Microbiome. 2019;7:47. doi: 10.1186/s40168-019-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Y., Liu X., Lv X., et al. Watermass properties and deep currents in the northern Yap Trench observed by the submersible Jiaolong system. Deep Sea Res. Part I. 2018;139:27–42. [Google Scholar]
- 114.Fu L., Li D., Mi T., et al. Characteristics of the archaeal and bacterial communities in core sediments from southern Yap Trench via in situ sampling by the manned submersible Jiaolong. Sci. Total Environ. 2020;703:134884. doi: 10.1016/j.scitotenv.2019.134884. [DOI] [PubMed] [Google Scholar]
- 115.Liu R., Wang Z., Wang L., et al. Bulk and active sediment prokaryotic communities in the Mariana and Mussau Trenches. Front. Microbiol. 2020;11:1521. doi: 10.3389/fmicb.2020.01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nielsen J.G. Fishes from depths exceeding 6000 meters. Galathea Rep. 1964;7:113–124. [Google Scholar]
- 117.Wang K., Shen Y., Yang Y., et al. Morphology and genome of a snailfish from the Mariana Trench provide insights into deep-sea adaptation. Nat. Ecol. Evol. 2019;3:823–833. doi: 10.1038/s41559-019-0864-8. [DOI] [PubMed] [Google Scholar]
- 118.Gerringer M.E., Popp B.N., Linley T.D., et al. Comparative feeding ecology of abyssal and hadal fishes through stomach content and amino acid isotope analysis. Deep Sea Res. Part I. 2017;121:110–120. [Google Scholar]
- 119.Gerringer M. On the success of the hadal snailfishes. Integrative Organismal Biology. 2019;1:1–18. doi: 10.1093/iob/obz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gerringer M.E., Drazen J.C., Linley T.D., et al. Distribution, composition and functions of gelatinous tissues in deep-sea fishes. R. Soc. Open Sci. 2017;4:171063. doi: 10.1098/rsos.171063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blankenship L.E., Yayanos A.A., Cadien D.B., et al. Vertical zonation patterns of scavenging amphipods from the hadal zone of the Tonga and Kermadec Trenches. Deep Sea Res. Part I. 2006;53:48–61. [Google Scholar]
- 122.Blankenship L.E., Levin L.A. Extreme food webs: Foraging strategies and diets of scavenging amphipods from the ocean’s deepest 5 kilometers. Limnol. Oceanogr. 2007;52:1685–1697. [Google Scholar]
- 123.Li D., Zhao J., Liu C., et al. Advances of living environment characteristics and biogeochemical processes in the hadal zone. J. China Univ. Geosci. 2018;43:1–20. (In Chinese with English abstract) [Google Scholar]
- 124.Lemche H., Hansen B., Madsen F.J., et al. hadal life as analysed from photographs. Vidensk Meddr Dansk Naturh Foren. 1976;139:263–336. [Google Scholar]
- 125.George R.Y., Higgins R.P. Eutrophic hadal benthic community in the Puerto Rico Trench. Ambio Special Report. 1979;6:51–58. [Google Scholar]
- 126.Smith C., Demopoulos A. In: Ecosystems of the Deep Oceans, Ecosystems of the World. Tyler P.A., editor. Elsevier; 2003. The deep Pacific Ocean floor; pp. 179–218. [Google Scholar]
- 127.Kobayashi H., Hatada Y., Tsubouchi T., et al. The hadal Amphipod Hirondellea gigas possessing a unique cellulase for digesting wooden debris buried in the deepest seafloor. PLoS One. 2012;7:e42727. doi: 10.1371/journal.pone.0042727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kobayashi H., Nagahama T., Arai W., et al. Polysaccharide hydrolase of the hadal zone amphipods Hirondellea gigas. Biosci., Biotechnol., Biochem. 2018;82:1123–1133. doi: 10.1080/09168451.2018.1459178. [DOI] [PubMed] [Google Scholar]
- 129.Lan Y., Sun J., Tian R., et al. Molecular adaptation in the world's deepest-living animal: Insights from transcriptome sequencing of the hadal amphipod Hirondellea gigas. Mol. Ecol. 2017;26:3732–3743. doi: 10.1111/mec.14149. [DOI] [PubMed] [Google Scholar]
- 130.Yancey P.H., Gerringer M.E., Drazen J.C., et al. Marine fish may be biochemically constrained from inhabiting the deepest ocean depths. Proc. Natl. Acad. Sci. U S A. 2014;111:4461–4465. doi: 10.1073/pnas.1322003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jambeck J.R., Geyer R., Wilcox C., et al. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 132.Peng X., Dasgupta S., Zhong G., et al. Large debris dumps in the northern South China Sea. Mar. Pollut. Bull. 2019;142:164–168. doi: 10.1016/j.marpolbul.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 133.Lee J.J. Ocean's deep, dark trenches to get their moment in the spotlight. Science. 2012;336:141–143. doi: 10.1126/science.336.6078.141. [DOI] [PubMed] [Google Scholar]
- 134.Miyake H., Shibata H., Furushima Y. In: Omori K., Guo X., Yoshie N., et al., editors. Vol. 5. TERRAPUB; 2011. Deep-sea litter study using deep-sea observation tools; pp. 261–269. (Interdisciplinary Studies on Environmental Chemistry). [Google Scholar]
- 135.Tsang Y.Y., Mak C.W., Liebich C., et al. Microplastic pollution in the marine waters and sediments of Hong Kong. Mar. Pollut. Bull. 2017;115:20–28. doi: 10.1016/j.marpolbul.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 136.Van Cauwenberghe L., Vanreusel A., Mees J., et al. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013;182:495–499. doi: 10.1016/j.envpol.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 137.Chen M., Du M., Jin A., et al. Forty-year pollution history of microplastics in the largest marginal sea of the western Pacific. Geochem. Perspect. Lett. 2020;13:42–47. [Google Scholar]
- 138.Fischer V., Elsner N.O., Brenke N., et al. Plastic pollution of the Kuril–Kamchatka Trench area (NW pacific) Deep Sea Res., Part II. 2015;111:399–405. [Google Scholar]
- 139.Jamieson A.J., Brooks L.S.R., Reid W.D.K., et al. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci. 2019;6:180667. doi: 10.1098/rsos.180667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.GESAMP Sources, fate and effects of microplastics in the marine environment: part two of a global assessment. Kershaw P.J., Rochman C.M., editors. Rep. Stud. GESAMP. 2016;93:220. [Google Scholar]
- 141.Desforges J.-P.W., Galbraith M., Dangerfield N., et al. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014;79:94–99. doi: 10.1016/j.marpolbul.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 142.Goldstein, M., Rosenberg, M., and Cheng, L. (2012). Increased abundance and ecological implications of plastic microdebris in the North Pacific Subtropical Gyre. In 97th ESA Annual Convention.
- 143.Eriksen M., Maximenko N., Thiel M., et al. Plastic pollution in the South Pacific Subtropical Gyre. Mar. Pollut. Bull. 2013;68:71–76. doi: 10.1016/j.marpolbul.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 144.Courtene-Jones W., Quinn B., Gary S.F., et al. Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ. Pollut. 2017;231:271–280. doi: 10.1016/j.envpol.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 145.Bergmann M., Wirzberger V., Krumpen T., et al. High quantities of microplastic in Arctic deep-sea sediments from the HAUSGARTEN observatory. Environ. Sci. Technol. 2017;51:11000–11010. doi: 10.1021/acs.est.7b03331. [DOI] [PubMed] [Google Scholar]
- 146.Kanhai L.D.K., Gårdfeldt K., Lyashevska O., et al. Microplastics in sub-surface waters of the Arctic Central Basin. Mar. Pollut. Bull. 2018;130:8–18. doi: 10.1016/j.marpolbul.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 147.Woodall L.C., Sanchez-Vidal A., Canals M., et al. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014;1:140317. doi: 10.1098/rsos.140317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jamieson A., Malkocs T., Piertney S., et al. Bioaccumulation of persistent organic pollutants in the deepest ocean fauna. Nat. Ecol. Evol. 2017;1:0051. doi: 10.1038/s41559-016-0051. [DOI] [PubMed] [Google Scholar]
- 149.Kaiser J. The dirt on ocean garbage patches. Science. 2010;328:1506. doi: 10.1126/science.328.5985.1506. [DOI] [PubMed] [Google Scholar]
- 150.Wang N., Shen C., Sun W., et al. Penetration of bomb 14C into the deepest ocean trench. Geophys. Res. Lett. 2019;46:5413–5419. [Google Scholar]
- 151.Lallas P. The Stockholm Convention on Persistent Organic Pollutants. Am. J. Int. Law. 2001;95:692–708. [Google Scholar]
- 152.Aichner B., Bussian B., Lehnik-Habrink P., et al. Levels and spatial distribution of persistent organic pollutants in the environment: a case study of German forest soils. Environ. Sci. Technol. 2013;47:12703–12714. doi: 10.1021/es4019833. [DOI] [PubMed] [Google Scholar]
- 153.Ma Y., Halsall C.J., Crosse J.D., et al. Persistent organic pollutants in ocean sediments from the North Pacific to the Arctic Ocean. J. Geophys. Res. Oceans. 2015;120:2723–2735. [Google Scholar]
- 154.Combi T., Miserocchi S., Langone L., et al. Polychlorinated biphenyls (PCBs) in sediments from the western Adriatic Sea: Sources, historical trends and inventories. Sci. Total Environ. 2016;562:580–587. doi: 10.1016/j.scitotenv.2016.04.086. [DOI] [PubMed] [Google Scholar]
- 155.Liu L., Tang J., Zhong G., et al. Spatial distribution and seasonal variation of four current-use pesticides (CUPs) in air and surface water of the Bohai Sea. China. Sci. Total Environ. 2018;621:516–523. doi: 10.1016/j.scitotenv.2017.11.282. [DOI] [PubMed] [Google Scholar]
- 156.Cui J., Yu Z., Mi M., et al. Occurrence of halogenated organic pollutants in hadal trenches of the western Pacific Ocean. Environ. Sci. Technol. 2020;54:15821–15828. doi: 10.1021/acs.est.0c04995. [DOI] [PubMed] [Google Scholar]
- 157.Dasgupta S., Peng X., Chen S., et al. Toxic anthropogenic pollutants reach the deepest ocean on Earth. Geochem. Perspect. Lett. 2018;7:22–26. [Google Scholar]
- 158.Phillips D.J. In: PCBs and the Environment Vol. II. Waid J.S., editor. CRC Press; 1986. Use of organisms to quantify PCBs in marine and estuarine environments; pp. 127–181. [Google Scholar]
- 159.Li Z., Fitzgerald N.M., Albert Z., et al. Contrasting mechanisms of metoprolol uptake on kaolinite and talc. Chem. Eng. J. 2015;272:48–57. [Google Scholar]
- 160.Bergquist B.A., Blum J.D. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science. 2007;318:417–420. doi: 10.1126/science.1148050. [DOI] [PubMed] [Google Scholar]
- 161.Sun R., Yuan J., Sonke J., et al. Methylmercury produced in upper oceans accumulates in deep Mariana Trench fauna. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-17045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Blum J.D., Drazen J.C., Johnson M.W., et al. Mercury isotopes identify near-surface marine mercury in deep-sea trench biota. Proc. Natl. Acad. Sci. U S A. 2020;117:29292–29298. doi: 10.1073/pnas.2012773117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Burger J., Gochfeld M., Burke S., et al. Do scientists and fishermen collect the same size fish? Possible implications for exposure assessment. Environ. Res. 2006;101:34–41. doi: 10.1016/j.envres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 164.Yang J., Cui Z., Dada O.A., et al. Distribution and enrichment of trace metals in surface marine sediments collected by the manned submersible Jiaolong in the Yap Trench, northwest Pacific Ocean. Mar. Pollut. Bull. 2018;135:1035–1041. doi: 10.1016/j.marpolbul.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 165.Welty C.J., Sousa M.L., Dunnivant F.M., et al. High-density element concentrations in fish from subtidal to hadal zones of the Pacific Ocean. Heliyon. 2018;4:e00840. doi: 10.1016/j.heliyon.2018.e00840. [DOI] [PMC free article] [PubMed] [Google Scholar]