Figure 1.
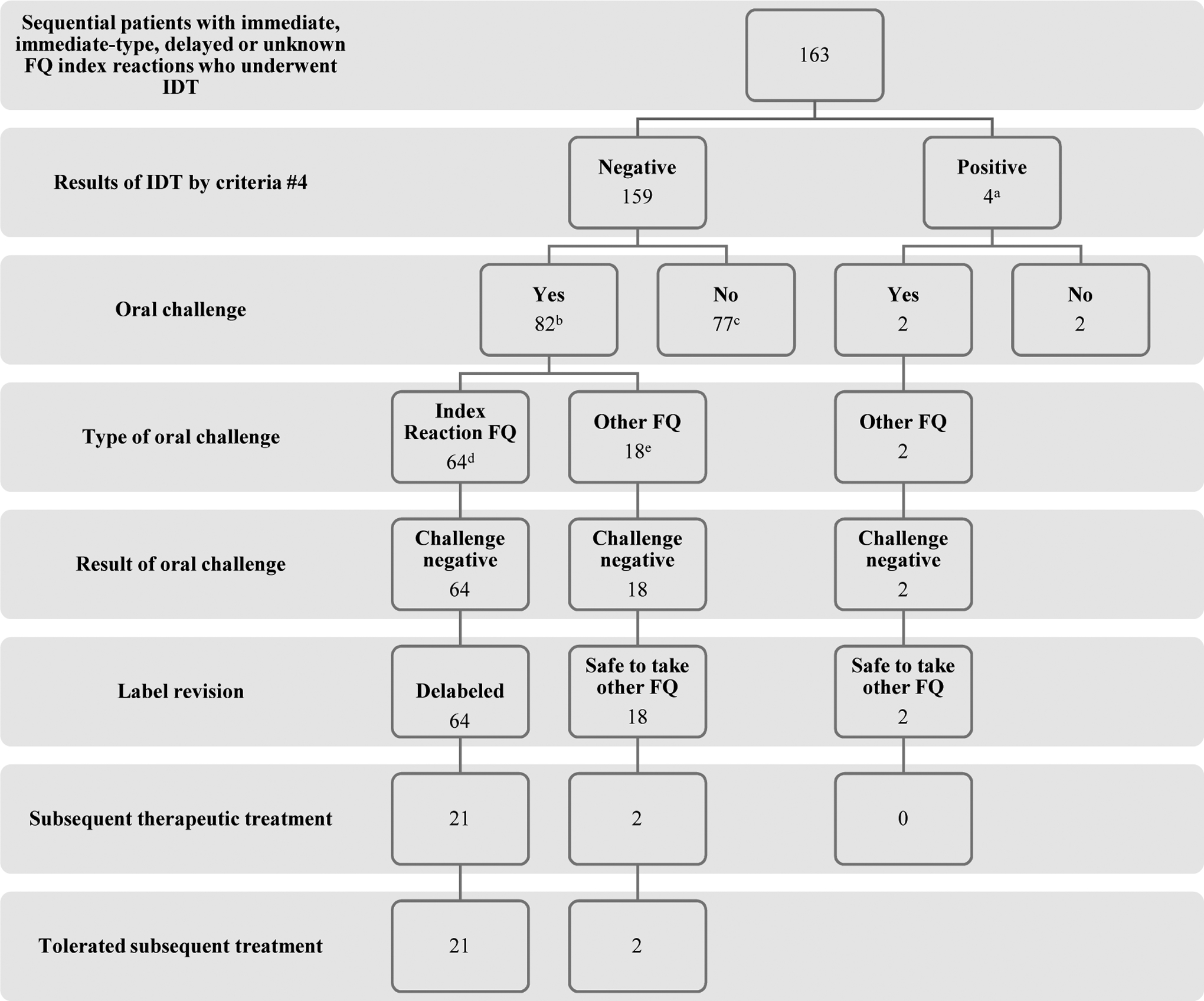
Flowchart of the study. FQ, fluoroquinolone; IDT, intradermal testing
a96/163 (59%) were IDT positive by criteria #1; 53/163 (33%) by criteria #2; and 36/163 (22%) by criteria #3 at either 0.005 mg/L or 0.025 mg/mL for at least 1 FQ
b47/82 (57%) were positive by criteria #1; 27/82 (29%) by criteria #2; and 29/82 (35%) by criteria #3 at either 0.005 mg/L or 0.025 mg/mL for at least 1 FQ. For greater detail on index reaction type, severity, and FQ; oral challenge FQ; subsequent treatment FQ, please see Table E2 (available in this article’s Online Repository at www.jaci-inpractice.org)
cThese 77 patients did not undergo OC as a result of time constraints in clinic due to our prioritized testing of beta-lactam and sulfa allergies first. All of these 77 patients, if they were to return to clinic in the future, would qualify for FQ oral challenge.
d30 were to ciprofloxacin, 17 to levofloxacin, 2 to moxifloxacin and 15 to at least 1 index FQ in patient with allergy history to ≥ 2 FQ.
eThe 18 patients who underwent oral challenge to an alternative FQ did so earlier on in the development of this FQ testing strategy due to a cautious evolution of our routine clinical care. Of these 18 patients, 4 reported an index reaction history of anaphylaxis or multisystem involvement compatible with anaphylaxis and 2 reported an immediate reaction consisting of urticaria.
