Figure 7.
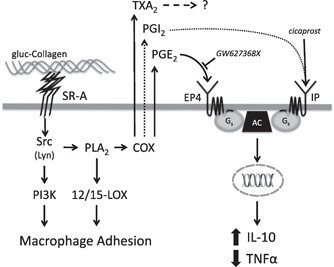
The role of PG in SR‐A‐mediated signaling during macrophage adhesion. SR‐A‐mediated macrophage adhesion involves the activation of a Src kinase, PI3K, PLA2, and 12/15‐LOX (Fig. 1 and refs. [16, 18]). Previous studies show that the Src kinase Lyn mediates the activation of PI3K during SR‐A‐mediated adhesion [18]. The current results (Fig. 2) indicate that PLA2 is also activated by a Src kinase (e.g., Lyn) and that COX‐derived products (PGE2, PGI2, and TXA2) are produced following the PLA2‐dependent generation of AA (Fig. 4). PGE2 suppresses TNF‐α and promotes IL‐10 production via binding to the EP4 receptor (Figs. 5 and 6), which elicits responses via Gs and adenylyl cyclase (AC). Although activation of the Gs‐coupled IP receptor can mediate similar effects on cytokine production (dotted lined), the amount of PGI2 produced during SR‐A‐mediated macrophage adhesion is not sufficient to elicit these responses. TXA2, which is also produced, has no effect on cytokine production during SR‐A‐mediated macrophage adhesion.
