Abstract
Leprosy or Hansen’s disease is a disabling infectious disease caused by Mycobacterium leprae. Reliance on the self-presentation of patients to the health services results in many numbers of leprosy cases remaining hidden in the community, which in turn results in a longer delay of presentation and therefore leading to more patients with disabilities. Although studies in Ethiopia show pockets of endemic leprosy, the extent of hidden leprosy in such pockets remains unexplored. This study determined the magnitude of hidden leprosy among the general population in Fedis District, eastern Ethiopia. A community-based cross-sectional study was conducted in six randomly selected leprosy-endemic villages in 2019. Health extension workers identified study participants from the selected villages through active case findings and household contact screening. All consenting individuals were enrolled and underwent a standardized physical examination for diagnosis of leprosy. Overall, 262 individuals (214 with skin lesions suspected for leprosy and 48 household contacts of newly diagnosed leprosy cases) were identified for confirmatory investigation. The slit skin smear technique was employed to perform a bacteriological examination. Data on socio-demographic characteristics and clinical profiles were obtained through a structured questionnaire. Descriptive statistics and binary logistic regression were used to assess the association between the outcome variable and predictor variables, and the P-value was set at 0.05. From the 268 individuals identified in the survey, 6 declined consent and 262 (97.8%) were investigated for leprosy. Fifteen cases were confirmed as leprosy, giving a detection rate of 5.7% (95%, CI: 3%, 9%). The prevalence of hidden leprosy cases was 9.3 per 10,000 of the population (15/16107). The majority (93.3%) of the cases were of the multi-bacillary type, and three cases were under 15 years of age. Three cases presented with grade II disability at initial diagnosis. The extent of hidden leprosy was not statistically different based on their sex and contact history difference (p > 0.05). High numbers of leprosy cases were hidden in the community. Active cases findings, and contact screening strategies, play an important role in discovering hidden leprosy. Therefore, targeting all populations living in leprosy pocket areas is required for achieving the leprosy elimination target.
Author summary
Leprosy, also called Hansen’s disease, is a neglected infectious disease leading to deformity and disability. Late presentation and hidden cases are the major risks of leprosy-associated disability. Although leprosy endemic pocket areas and grade II disability with a high proportion were reported in Ethiopia, studies on the burden of hidden leprosy cases are limited. Therefore, this study determined the extent of hidden leprosy cases among the general population in leprosy endemic settings in eastern Ethiopia through active case findings and contact tracing. In this community-based survey, leprosy-suspected individuals in the general population and household contacts of newly diagnosed patients with leprosy were included. Health extension workers, community-based health workers in Ethiopia, visited 16107 individuals in the selected villages and 214 leprosy suspects were enrolled in the study based on the clinical signs of leprosy suspects. Leprosy experts examined all leprosy suspects clinically and a skin slit sample was taken for bacteriological examination. After the confirmation of new cases, 48 of their households’ contacts were then examined by leprosy experts. Of 262 suspects and household contacts evaluated for leprosy, 15 hidden cases confirmed, giving an overall prevalence of 9.3 per 10, 000 population. Most of them were Multi-bacillary (MB) type, and one-fourth of them were younger than 15 years of age, and three cases presented with grade II disability. Hidden leprosy was not statistically associated with participants’ sex, age category, and contact history.
Introduction
Leprosy or Hansen’s disease is a disabling infectious disease caused by Mycobacterium leprae [1]. It is one of the neglected tropical diseases of public health importance [1,2]. Leprosy is endemic in poor countries where detection rates remain low despite availability of effective treatment [3]. Though there have been reductions of about 90% in the prevalence rate, transmission continues and remains a public health issue [4].
The global target to eliminate leprosy, a reduction in prevalence to <1 case per 10,000 population, was achieved in 2000[5] and the World Health Organization (WHO) had set a target to interrupt the transmission of leprosy globally by 2020 [6]. Leprosy nevertheless continues to be a public health problem in different parts of the world [7], with more than 200,000 new cases reported every year [8].
Although Ethiopia achieved the elimination target in 1999, it still has the second-highest disease burden in terms of leprosy in Sub-Saharan Africa (SSA) [9]. Between 2013 and 2015, 3,500 to 4,000 new leprosy cases were reported to the national tuberculosis and leprosy control program [10]. In 2019, the country reported 3,201 new leprosy patients, of whom 12.8% presented with a grade II disability, as reported by WHO [8]. Studies in Ethiopia also evidenced the persistent prevalence of childhood leprosy and disabilities with multibacillary (MB) cases in rural southern Ethiopia [11,12], which suggested the ongoing transmissions of the disease in the country [13].
Active case-findings strategies are essential for discovering hidden leprosy and are an important epidemiological tool to minimize cases, reduce incidences of disability due to leprosy, and reduce the transmission of M. leprae [14,15]. Moreover, the global leprosy strategy (2016–2020) promotes early case detection by the application of active case-finding and contact management in areas of higher endemicity [13].
However, in Ethiopia cases of leprosy are detected by examining patients attending health facilities (passive case detection)[16]. This passive case detection or self-reporting of patients in the integrated leprosy-control program results in increased hidden and undiagnosed leprosy cases in the community, leading to more deformities and disability [17].
While studies in Ethiopia revealed endemic leprosy pockets [18], the extent of hidden leprosy cases is rarely addressed [9]. Therefore, this study determined the magnitude of hidden and undiagnosed leprosy using house-to-house visits as a tool for active case detection and to evaluate the household contacts of leprosy in selected leprosy endemic districts in eastern Ethiopia in 2019.
Methods
Ethical consideration
This study was conducted according to the Helsinki Declaration and Ethiopian research regulations. The Institutional Health Research Ethics Review Committee (IHRERC) of the College of Health and Medical Sciences, Haramaya University, Ethiopia (ref no: IHRERC/152/2018) and the Armauer Hansen Research Institute Ethics Committee (ref no: P002/18 AHRI/ERC) approved the protocol. The coordinator informed all participants in advance about the purpose and time of the survey. Participants were given information on the objectives of the study, and informed consent was obtained in writing or by thumbprint. For those participants below 18 years of age, informed consent was obtained from a parent or legal guardian. To minimize the stigma, privacy was a priority during the examination of study participants. Participants participated voluntarily and withdraw from the study at any time without any consequences. Anonymity was ensured by only having participant identification numbers included during data collection.
Study setting, design and period
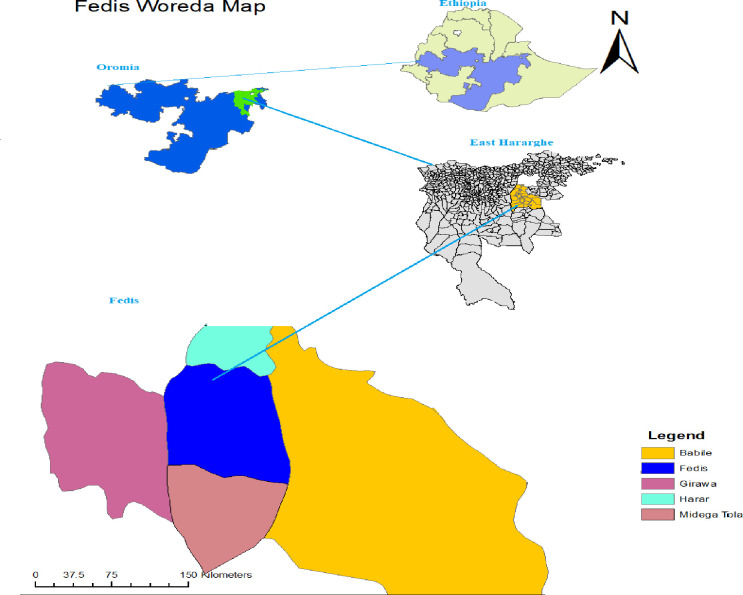
A community-based observational study was conducted in six leprosy endemic villages in the Fedis District between 5 July and 30 October 2019. Fedis is one of the high leprosy endemic districts located in East Hararghe Zone, Oromia Regional State, Eastern Ethiopia. It is located at 534 km East of Addis Ababa and 24km to the south of Harar (Fig 1).The district contains 19 rural and 2 urban villages with a total population estimated to be 133,382 persons. According to the zonal health office report, 57 and 47 new leprosy cases were receiving treatment in 2017 and 2018, resulting in a prevalence of 3.5 to 4.3 per 10,000 population (East Hararghe Zonal Health office, 2017 and 2018) (Zonal TB/Leprosy focal person communication).
Fig 1. Map showing the location of study site in Ethiopia 2019: Fedis district, drawn using ARCGIS version 10.1.
(source: "Natural Earth. (http://www.naturalearthdata.com/about/terms-of-use/).
Study population, and sampling
Since leprosy occurs in clusters, one large sample from a single area would not have been a reliable estimate of leprosy. Estimating the disease burden by conventional sampling procedure is difficult due to the large sample size requirement. Therefore, inverse sampling procedure was used [19,20]. Fedis District was selected among 12 high leprosy endemic districts in the East Hararghe Zone. From the District, six villages with a leprosy endemicity burden, with a total population of 35,673, were included randomly. All suspected cases and consenting individuals were screened through house-to-house visits and consecutively enrolled for leprosy diagnosis. Study criteria excluded those on multi-drug therapy at the initiation of the study and a person who lived for less than six months in the selected villages.
Data collection procedure and tools
Survey
Full village surveys of the selected villages were conducted, including all household members. Twelve trained health extension workers (HEWs) conducted the house-to-house visits to identify leprosy suspects. Using checklists, which was adopted from the national guideline, HEWs identified suspects by showing color photos of leprosy cases and asking if any household members had similar symptoms. Suspects were referred to the nearby health facility and examined by leprosy experts from Armauer Hansen Research Institute and the research team (researchers, health officers, and HEWs).
Physical examination
All individuals suspected of having leprosy underwent a standardized physical examination, as recommended by WHO and the national guidelines [21]. Briefly, the physical examination focused on examination of the skin from head to toe, including the front and back sides, the presence of skin lesions (patches or nodules), loss of sensation over the skin lesions (patches) using a “wisp of cotton wool”, and the number of skin lesions counted, if any. Palpation of the nerves was checked for cord enlargement and/or tenderness, and examination of eyes, hands and feet for any disabilities[22].
Bacteriological examination
According to the national guidelines, the slit skin smear examination was performed for questionable cases to confirm the diagnosis; and was also used for leprosy classification. One slide, with smears taken from two sites (ear lobes and active lesion), was collected for examination and evaluation for M. leprae (acid-fast bacilli)[16]. Accordingly, the principal investigators obtained skin smears for bacteriological examination. Briefly, slit-skin smears were taken from ear lobes and skin lesions from 43 study participants. The slit-skin smears made on the slide were stained by the Ziehl-Neelsen technique, using 1% carbol fuchsin, 1% acid-alcohol and 0.25% methyl blue. Under oil immersion objective, red acid-fast bacilli were observed, arranged singly or in groups (cigar-like bundles), and bound together by a lipid-like substance, forming glia. The criteria used for diagnosis and classification were based on the local leprosy control program and followed WHO guidelines as either paucibacillary (PB) or multibacillary (MB) type[21].
After confirmation of the leprosy diagnosis, the leprosy experts determined the degree of disability and initiated multi-drug therapy. The household contacts were then scheduled for screening by the research team. Leprosy experts or dermatologists then examined the household contacts. Suspects with other skin diseases were linked to the nearby health center.
Questionnaire
Structured questionnaires were administered to suspected cases and household contacts to obtain data on demographic characteristics and clinical history. Information related to leprosy diagnosis was obtained, including WHO classification of leprosy, disability grade, the clinical profile of individuals including BCG scar, contact history of leprosy, and any previous history of leprosy was documented.
Quality control
Health extension workers were trained in the clinical examination for leprosy diagnosis and how to refer suspected cases to the nearby health center for further investigation by leprosy experts. HEWs conducted an interview in the local language (Afan Oromo) and checklists were completed by face-to-face interviews for recruitment.
Study variables
The outcome variable was the magnitude of the hidden leprosy case. The independent variables include age, sex, occupation, residence, educational status, marital status, BCG scar, and contact history of a patient with leprosy.
Operational definition
Suspect is an individual who presented with pale or reddish patches (skin patch with discoloration) on the skin, painless swelling or lumps in the face and earlobes, loss of, or decreased sensation on the skin, numbness or tingling of the hands and/or feet, weakness of eyelids, hands or feet, painful and/or tender nerves, burning sensation in the skin or painless wounds or burns on the hands or feet [16].
A leprosy case is a person with one of the cardinal signs of leprosy, and who requires chemotherapy. The cardinal signs of leprosy are ONE of the following: hypo-pigmented skin lesion with definite loss of sensation, thickened (enlarged) peripheral nerve with or without tenderness, and/or the presence of acid-fast bacilli in a slit-skin smear [16].
The PB type is a patient who is skin smear negative and/or the number of skin lesions is 1–5 without demonstrated presence of bacilli in the smear [23].
The MB type is a patient who is skin smear positive and/or the number of skin lesions is more than five, with demonstrated presence of bacilli in the smear, irrespective of the number of skin lesions [23].
Physical disability in leprosy is defined by the WHO in three categories [24]: Grade 0: the absence of disability (no anesthesia) and no visible damage or deformities of eyes, hands and feet; Grade I disability: the loss of protective sensibility in the eyes, hands or feet, but no visible damage or deformities; and Grade II: the presence of deformities or visible damage to the eyes (lagophthalmos and/or ectropion, trichiasis, corneal opacity, difficulty counting fingers at 6 meters), visible damage on hands or feet (hand with ulcerations and/or traumatic, resorption, claw, fallen hand, ulcers; feet with trophic and/or traumatic injuries, resorption, claw, foot drop, ulcers, ankle contracture) [25].
Household contact is a family member or any person that who lived under the same roof with the index case for more than six months [26]. Co-prevalent leprosy is where the contacts diagnosed with leprosy at the first examination after the index case were diagnosed [27].
Data management and statistical analysis
Data were entered in Epi-Data version 3.1 and analyzed using STATA version 13.0. Descriptive statistics such as mean and percentages were used to describe the socio-demographic characteristics and the magnitude of hidden leprosy cases. Descriptive statistics such as mean and proportion and binary logistic regression analysis were used to assess the association between the dependent and predictor variables. The significant association was declared at p-value < 0.05.
Results
Demographic characteristics of the study participants
The HEW visited 16,107 individuals during a house-to-house survey and household contact (HHC) tracing. Of these, 268 were eligible, 262 (97.8%) of whom consented to participation and were enrolled in the study. Among the volunteers who were evaluated for leprosy, 214 participants were identified as suspects for leprosy during the house-to-house visit, and 48 were household contacts of newly diagnosed cases. The mean (+ SD) age of the participants was 26.9 (±15.2) years. About 45% of the participants were female and 62% were rural residents. About half (48%) of the participants had no formal education (Table 1).
Table 1. Distribution of demographic and clinical condition among participants with or without leprosy in Fedis District, 2019(n = 262).
| Variables | Hidden leprosy | |||||
|---|---|---|---|---|---|---|
| Negative | Positive | Total | ||||
| Sex | Number | % | Number | (%) | Number | % |
| Male | 134 | 92.4 | 11 | 7.6 | 145 | 55.3 |
| Female | 113 | 96.6 | 4 | 3.4 | 117 | 44.7 |
| Total | 262 | 100 | ||||
| Age category (in years) | ||||||
| < 15 | 60 | 95.2 | 3 | 4.8 | 63 | 24.1 |
| 15–30 | 99 | 94.3 | 6 | 5.7 | 105 | 40.1 |
| 31–45 | 66 | 97.1 | 2 | 2.9 | 68 | 25.9 |
| Above 45 | 22 | 84.6 | 4 | 15.4 | 26 | 9.9 |
| Total | 262 | 100 | ||||
| Educational status | ||||||
| No formal education | 122 | 96.8 | 4 | 3.2 | 126 | 48.1 |
| Literate | 125 | 91.9 | 11 | 8.1 | 136 | 51.9 |
| Total | 262 | 100 | ||||
| Marital status | ||||||
| Single | 93 | 92.1 | 8 | 7.9 | 101 | 38.6 |
| Married | 154 | 95.6 | 7 | 4.4 | 161 | 61.4 |
| Total | 262 | 100 | ||||
| Occupation | ||||||
| Farmer | 139 | 95.2 | 7 | 4.8 | 146 | 55.7 |
| Employed | 15 | 100.0 | 0 | 0.0 | 15 | 5.7 |
| Unpaid | 93 | 92.1 | 8 | 7.9 | 101 | 38.6 |
| Total | 262 | 100 | ||||
| Residence | ||||||
| Rural | 155 | 95.7 | 7 | 4.3 | 162 | 61.8 |
| Urban | 92 | 92.0 | 8 | 8.0 | 100 | 38.2 |
| Total | 262 | 100 | ||||
Prevalence of hidden leprosy
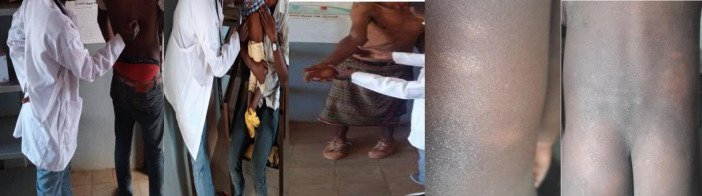
During active case-finding through the house-to-house visits, 214 suspects were evaluated both clinically and/or bacteriologically (Fig 2). Thirty (14%) of the suspects had histories of contact with treated leprosy patients. Of the 214 suspects, 11 leprosy cases were confirmed, giving a detection rate of 5.1% (95%, CI = 2%, 9%). Among the newly confirmed leprosy cases, one patient had a prior history of leprosy (relapse case) and three cases had a contact history with a treated leprosy patient. The majority (90.9%) of cases were MB type leprosy, and two of them presented with grade II disability. Most (63.6%) of cases were farmers and 81.8% were male.
Fig 2. Physical examination of individuals suspected for leprosy based on cardinal signs at leprosy clinic, Fedis district, 2019.
Following the confirmation of 11 new cases, leprosy experts and dermatologists examined 48 HHCs through contact management strategy. Among the 48 HHCs, four new leprosy cases (co-prevalent cases) were confirmed, giving an 8.3% detection rate (95%, CI = 2%, 19%). Among the co-prevalent cases, all of them were MB and two cases were under 15 years of age.
By considering both suspects and HHCs evaluations, 15 participants were found to be leprosy cases, giving a detection rate of 5.7% (95%, CI: 3%, 9%). This yields a total population-based prevalence of hidden leprosy to be 9.3 per 10,000 population. The majority 14(93.3%) of the newly diagnosed hidden cases were MB, and three cases demonstrated grade II disability. Among the newly diagnosed hidden cases, three were under 15 years of age and about one-fourth were female.
The extent of hidden leprosy was not statistically different based on their age category and contact history difference (p > 0.05). Furthermore, in the binary logistic regression analysis, the detection rate of hidden leprosy cases was not statistically different based on their sex difference (P>0.05) (Table 2).
Table 2. Association between dependent and predictor variables among study participants in leprosy endemic setting, Fedis District, 2019(n = 262).
| Variables | OR | [95%Conf. Interval] | p-value | AOR* | [95%Conf. Interval] | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | 1 | |||||||
| Female | 0.43 | 0.13 | 1.39 | 0.15 | 0.45 | 0.13 | 1.47 | 0.18 | |
| Marital status | Single | 1 | |||||||
| Married | 0.52 | 0.18 | 1.50 | 0.23 | 0.63 | 0.17 | 2.30 | 0.48 | |
| Educational Status | No formal education | 1 | |||||||
| literate | 2.68 | 0.83 | 8.65 | 0.09 | 1.73 | 0.41 | 7.29 | 0.45 | |
| Residence | Rural | ||||||||
| Urban | 1.92 | 0.67 | 5.48 | 0.22 | 1.72 | 0.55 | 5.35 | 0.34 | |
AOR* = adjusted odds ratio, variables in the final model
Discussion
This study revealed the high prevalence of hidden leprosy in the general population. All co-prevalent patients were detected without having significant neuronal or visible physical damage at the initial stage of screening. Hidden leprosy was not associated with participants’ demographic characteristics and contact histories. Therefore, the presence of pockets of high endemicity with a high prevalence rate of 9.3 per 10,000 population points to the arduous journey ahead for leprosy elimination in Ethiopia.
Ethiopia, with the introduction of multi-drug treatment (MDT), achieved the elimination target at the national level with a record of 0.3 per 10,000 population in 2018 [22]. Our finding is higher than the national prevalence and that of Gambella regional state, which was 2.4 per 10,000 in 2016 [22]. We used an active case detection strategy, compared to the above-mentioned lower estimates, which used passive case detection. However, the national leprosy control program recommended the voluntary self-reporting (passive case detection) strategy. Moreover, the higher prevalence is evidence for the poor performance of passive case detection compared with active case findings [28] and active case finding is an important epidemiological tool to minimize hidden leprosy cases [15].
In this study all co-prevalent patients among HHCs were detected without having a significant neuronal or visible physical damage at the initial stage of screening. This is an indication of the feasibility and contribution of active case-finding programs to promote early case detection by tracking HHCs [13,29]. We found that one in five cases presented with grade II disability on diagnosis, showing a prolonged delay in health-seeking. This is in harmony with the research conducted in Addis Ababa, where the proportion of grade II disability among new leprosy cases was 23.7% [30]. This finding is higher than the national report of 13.6% in 2016 [22]. The higher proportion of grade II disability in the current study supports the late case presentation and ongoing transmission of leprosy [26,31]. It also reflects inadequate monitoring in the national leprosy control program [13] and the ongoing transmission of leprosy has not been interrupted [26]. Unfavorable attitude toward leprosy among the community in the same study setting [32] contributes to late presentation [33].
The proportion of childhood prevalence (20%) in this study is higher than the national prevalence (11.7%) and that of Oromia regional state (13.3%) [22]. The presence of childhood leprosy among new cases suggested the existence of the active source of infection [34] and high ongoing transmission of the disease in the community [22]. The higher proportion of childhood leprosy also a late performance indicator of the national leprosy control program [35].
This study revealed that hidden leprosy is not significantly associated with participants’ contact history with leprosy and their sex difference. Similar findings have been reported in other countries [15,36]. All study participants resided in shared vulnerable areas with high leprosy endemicity villages and the same environmental exposure status [37,38]. Likewise, more than half of the community in Fedis District was food insecure [39]. Food shortage is shown as an important poverty-related predictor of the clinical manifestation of leprosy and the greatest risk [40]. Therefore, they have the greatest risk of leprosy [41]. Hence, a higher prevalence of leprosy is expected in this district. Also, the unfavorable attitude in the general community in Fedis District and the stigma favors the hiding of patients from the diagnosis, irrespective of their sex and contact history [32,42].
Strength and limitation of the study
This community-based active survey evidences the hidden leprosy cases that were missed by passive case detection in an endemic-leprosy setting. This study discovers leprosy patients who didn’t seek health care before the inclusion. These leprosy patients are hidden within the general population and risk for themselves and others. All examinations of suspects were done in accordance with the national guidelines for leprosy diagnosis. Experienced leprosy experts and dermatologists performed clinical examinations. Learning from the successes of other disease prevention and improved health service utilization or health-care seeking through the deployment of health extension program in Ethiopia[43–45], we used the trained health extension workers as data collectors to discover hidden leprosy. Using the existing health extension programs in a context of limited resources is more workable and provides more reliable data.
The inclusion of suspects was based on questioning individuals according to the leprosy symptoms; individuals cannot recognize painless symptoms or do not report to the HEW during the house-to-house visits due to fear of stigma [33,46](selection bias). However, the colored picture used during the interview helped in recognizing symptoms.
Conclusions and recommendations
Conclusions
The overall prevalence of hidden leprosy is higher than the national and regional figures. An active finding and tracing of HHC in regions where leprosy is highly prevalent, like Fedis District, is an important strategy to promote early diagnosis, minimize hidden leprosy and prevent severe outcomes. The prevalence of hidden leprosy was not significantly different based on the contact history and demographic characteristics of the participants.
Recommendations
An outreach activity of active case-finding targeting all age and sex group populations in leprosy pocket areas is crucial to stop leprosy and its complications.
It is important to develop a framework that incorporates leprosy case-finding and HHC tracing strategies in the implementation of the health extension program.
Further studies considering larger sample size and different study design need to be undertaken to identify potential factors associated with hidden leprosy.
Acknowledgments
The authors would like to acknowledge the study participants, the district health office and data collectors for Haramaya University and AHRI their support in the study. Our great appreciation also goes to project supervisors and colleagues for their support.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the manuscript.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hay RJ. Skin Disease in the Tropics and the Lessons that can be Learned from Leprosy and Other Neglected Diseases. Acta dermato-venereologica. 2020;100(9):adv00113. Epub 2020/03/25. doi: 10.2340/00015555-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abeje T, Negera E, Kebede E, Hailu T, Hassen I. Performance of general health workers in leprosy control activities at public health facilities in Amhara and Oromia States, Ethiopia. BMC Health Services Research. 2016;16(122). doi: 10.1186/s12913-016-1329-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talhari S, Grossi MAdF, Oliveira MLWd, Gontijo B, Talhari C, Penna GO. Hansen’s disease: a vanishing disease? Memórias do Instituto Oswaldo Cruz. 2012;107:13–6. doi: 10.1590/s0074-02762012000900003 [DOI] [PubMed] [Google Scholar]
- 4.Brito e Cabral P, Júnior JE, de Macedo AC, Alves AR, Gonçalves TB, Brito e Cabral TC, et al. Anti-PGL1 salivary IgA/IgM, serum IgG/IgM, and nasal Mycobacterium leprae DNA in individuals with household contact with leprosy. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2013;17(11):e1005–10. Epub 2013/07/23. doi: 10.1016/j.ijid.2013.05.011 . [DOI] [PubMed] [Google Scholar]
- 5.Rinaldi A. The Global Campaign to Eliminate Leprosy. PLoS Med. 2005;2(12):e341. doi: 10.1371/journal.pmed.0020341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blok D, De Vlas S, Richardus J. Global elimination of leprosy by 2020: are we. Parasites & Vectors. 2015;8(548). doi: 10.1186/s13071-015-1143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arif T, Amin SS, Adil M, Dorjay K, Raj D. Leprosy in the post-elimination era: a clinico-epidemiological study from a northern Indian tertiary care hospital. Acta dermatovenerologica Alpina, Pannonica, et Adriatica. 2019;28(1):7–10. Epub 2019/03/23. . [PubMed] [Google Scholar]
- 8.WHO. Weekly epidemiological record. 2020. p. 417–40. [Google Scholar]
- 9.Deribe K, Meribo K, Gebre T, Hailu A, Ali A, Aseffa A, et al. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasites & vectors. 2012;5:240–. doi: 10.1186/1756-3305-5-240 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GLRA-ETHIOPIA. Annual Master Report. 2015. [Google Scholar]
- 11.Ramos Rincón J, Reyes Rabell F, Lemma D, Tesfamariam A, Belinchón I. The burden of leprosy in children and adolescents in rural Southern Ethiopia. Paediatrics and international child health. 2013;34. [DOI] [PubMed] [Google Scholar]
- 12.Baye S. Leprosy in Ethiopia: Epidemiological trends from 2000 to 2011. Advances in life sciences. 2015;2:31–44. [Google Scholar]
- 13.WHO. Global Leprosy Strategy, 2016–2020: Accelerating towards a leprosy-free world. India: World Health Organization. 2016. [Google Scholar]
- 14.Kumar M, Padmavathi S, Shivakumar M, Charles U, Appalanaidu M. Hidden leprosy cases in tribal population groups and how to reach them through a collaborative effort. Lepr Rev. 2015;86:328–34. [PubMed] [Google Scholar]
- 15.Moura M, Dupnik K, Sampaio G, Nobrega P, Jeronimo A, do Nascimento-Filho J, et al. Active Surveillance of Hansen’s Disease (Leprosy): Importance for Case Finding among Extra-domiciliary Contacts. PLoS Negl Trop Dis 2013;7(3):e2093. doi: 10.1371/journal.pntd.0002093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDRE MoH. Guidelines for Clinical and Programmatic Management of TB, Leprosy and TB/HIV in Ethiopia. Fifth Edition ed ed2012.
- 17.Giri V, Bhagat V, Baviskar S, Showkath M. Achieving Integration through Leprosy Case Detection Campaign (LCDC). International Journal of Biomedical Research 2017;8:38–41. 10.7439/ijbr. [DOI] [Google Scholar]
- 18.Bobosha K, Van Der Ploeg-Van Schip JJ, Zewdie M, Sapkota BR, Hagge DA, Franken KL, et al. Immunogenicity of Mycobacterium leprae unique antigens in leprosy endemic populations in Asia and Africa. Lepr Rev. 2011;82(4):445–58. Epub 2012/03/24. . [PubMed] [Google Scholar]
- 19.Katoch K, Aggarwal A, Yadav V, Pandey A. National sample survey to assess the new case disease burden of leprosy in India. Indian J Med Res 2017;146(5):585–605. doi: 10.4103/ijmr.IJMR_1496_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggarwal A, Pandey A. Inverse sampling to study disease burden of leprosy. The Indian journal of medical research. 2010;132:438–41. Epub 2010/10/23. . [PubMed] [Google Scholar]
- 21.WHO. Diagnosis of Leprosy. Leprosy Elimination: available at https://www.who.int/lep/diagnosis/en/ Accessed: April 15, 2018. 2018.
- 22.FDRE MoH. NATIONAL STRATEGIC PLAN TUBERCULOSIS AND LEPROSY CONTROL 2013/14–2020: With update–for (2018-20/21). 2017.
- 23.WHO. Guidelines for the diagnosis, treatment and prevention of leprosy: available at https://apps.who.int/iris/bitstream/handle/10665/274127/9789290226383-eng.pdf?ua=1. In:. ROfS-EA, editor. 2018.
- 24.Brandsam J, Wim H, Brakel V. WHO disability grading:operational definition. Lepr Rev. 2003;74:366–73. [PubMed] [Google Scholar]
- 25.WHO. Global strategy for further reducing the leprosy burden and sustaining leprosy control activities (2006–2010): Operational Guidelines. In:. ROfS-EA, editor. 2006. [PubMed]
- 26.Romero-Montoya M, Beltran-Alzate JC, Cardona-Castro N. Evaluation and Monitoring of Mycobacterium leprae Transmission in Household Contacts of Patients with Hansen’s Disease in Colombia. PLOS Neglected Tropical Diseases. 2017;11(1):e0005325. doi: 10.1371/journal.pntd.0005325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sales A, Ponce L, Duppre N, Hacker M, Nery J, Sarno E, et al. Leprosy among Patient Contacts: A Multilevel Study of Risk Factors. PLoS Negl Trop Dis. 2011;5(3):e1013. doi: 10.1371/journal.pntd.0001013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letta T. Challenges in implementation of the current strategies.https://www.who.int/docs/default-source/ntds/leprosy/global-consultation-on-global-leprosy-strategy-2021-2030/05-challenges-ethiopia.pdf. In: Global Leprosy Consultation M, editor. 2020. [Google Scholar]
- 29.Wang N, Chu T, Li F, Wang Z, Liu D, Chen M, et al. The role of an active surveillance strategy of targeting household and neighborhood contacts related to leprosy cases released from treatment in a low-endemic area of China. PLoS Negl Trop Dis 2020;14(8):e0008563. doi: 10.1371/journal.pntd.0008563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shumet T, Demissie M, Bekele Y. Prevalence of Disability and Associated Factors among Registered Leprosy Patients in All Africa Tb and Leprosy Rehabilitation and Training Centre (ALERT), Addis Ababa, Ethiopia. Ethiop J Health Sci 2015;25(4). doi: 10.4314/ejhs.v25i4.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith W, Brakel W, Gillis T, Saunderson P, Richardus J. The Missing Millions: A Threat to the Elimination of Leprosy. PLoS Negl Trop Dis 2015; 9(4). doi: 10.1371/journal.pntd.0003658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urgesa K, Bobosha K, Seyoum B, Geda B, Weldegebreal F, Mihret A, et al. Knowledge of and Attitude Toward Leprosy in a Leprosy Endemic District, Eastern Ethiopia: A Community-Based Study. Risk Management and Healthcare Policy. 2020;13:1069–77. doi: 10.2147/RMHP.S254625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Da Silva Souza C, Bacha J. Delayed diagnosis of leprosy and the potential role of educational activities in Brazil. Lepr Rev. 2003;74:249–58. [PubMed] [Google Scholar]
- 34.Barreto JG, Frade MAC, Bernardes Filho F, da Silva MB, Spencer JS, Salgado CG. Leprosy in Children. Current Infectious Disease Reports. 2017;19(6):23. doi: 10.1007/s11908-017-0577-6 [DOI] [PubMed] [Google Scholar]
- 35.Babu A, Bhat M, Jayaraman J. Childhood leprosy in the postelimination era: A vision achieved or a concern growing at large. Indian J Paediatr Dermatol. 2018;19:26–30. [Google Scholar]
- 36.Liu Y, Yu M, Ning Y, Wang H. A study on gender differences in newly detected leprosycases in Sichuan, China, 2000–2015. International Journal of Dermatology. 2018; 57:1492–9. doi: 10.1111/ijd.14148 [DOI] [PubMed] [Google Scholar]
- 37.Serrano-Coll H, Mora H, Beltrán C, Duthie M, Cardona-Castro N. Social and environmental conditions related to Mycobacterium leprae infection in children and adolescents from three leprosy endemic regions of Colombia. BMC Infectious Diseases. 2019:19:520. doi: 10.1186/s12879-019-4120-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turankar R, Lavania M, Singh M, Siva Sai K, Jadhav R. Dynamics of Mycobacterium leprae transmission in environmental context: deciphering the role of environment as a potential reservoir. Infect Genet Evol 2012;12:121–6. doi: 10.1016/j.meegid.2011.10.023 . [DOI] [PubMed] [Google Scholar]
- 39.Mulugeta M, Tiruneh G, Alemu ZA. Magnitude and associated factors of household food insecurity in Fedis Woreda East Hararghe zone, Oromia region, Ethiopia. Agriculture & Food Security. 2018;7(1):3. doi: 10.1186/s40066-017-0140-6 [DOI] [Google Scholar]
- 40.Feenstra SG, Nahar Q, Pahan D, Oskam L, Richardus JH. Recent food shortage is associated with leprosy disease in Bangladesh: a case-control study. PLoS neglected tropical diseases. 2011;5(5):e1029–e. doi: 10.1371/journal.pntd.0001029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nery J, Ramond A, Pescarini J, Alves A, Strina A, Ichihara M, et al. Socioeconomic determinants of leprosy new case detection in the 100 Million Brazilian Cohort: a population-based linkage study. Lancet Glob Health. 2019;7:e1226–36. doi: 10.1016/S2214-109X(19)30260-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cairns W, Smith S. Leprosy: making good progress but hidden challenges remain. Indian J Med Res. 2013;137:1–3. [PMC free article] [PubMed] [Google Scholar]
- 43.Assefa Y, Gelaw YA, Hill PS, Taye BW, Van Damme W. Community health extension program of Ethiopia, 2003–2018: successes and challenges toward universal coverage for primary healthcare services. Globalization and Health. 2019;15(1):24. doi: 10.1186/s12992-019-0470-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medhanyie A, Spigt M, Kifle Y, Schaay N, Sanders D, Blanco R, et al. The role of health extension workers in improving utilization of maternal health services in rural areas in Ethiopia: a cross sectional study. BMC Health Services Research. 2012;12(1):352. doi: 10.1186/1472-6963-12-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weldemarium T, Getachew M. Role of Health Extension Worker in Tuberculosis Prevention and Control in Ethiopia: Systematic Review. American Journal of Life Sciences 2019;7(1):1–4. doi: 10.11648/j.ajls.20190701.11 [DOI] [Google Scholar]
- 46.Henry M, GalAn N, Teasdale K, Prado R, Amar H, Rays M, et al. Factors Contributing to the Delay in Diagnosis and Continued Transmission of Leprosy in Brazil–An Explorative, Quantitative, Questionnaire Based Study. PLoS Negl Trop Dis 2016;10(3):e0004542. doi: 10.1371/journal.pntd.0004542 [DOI] [PMC free article] [PubMed] [Google Scholar]