Visual Abstract
Keywords: hemodialysis, COVID-19, infection control
Abstract
Background and objectives
Patients receiving in-center hemodialysis treatment face unique challenges during the coronavirus disease 2019 (COVID-19) pandemic, specifically the need to attend for treatment that prevents self-isolation. Dialysis unit attributes and isolation strategies that might reduce dialysis center COVID-19 infection rates have not been previously examined.
Design, setting, participants, & measurements
We explored the role of variables, including community disease burden, dialysis unit attributes (size and layout), and infection control strategies, on rates of COVID-19 among patients receiving in-center hemodialysis in London, United Kingdom, between March 2, 2020 and May 31, 2020. The two outcomes were defined as (1) a positive test for infection or admission with suspected COVID-19 and (2) admission to the hospital with suspected infection. Associations were examined using a discrete time multilevel time-to-event analysis.
Results
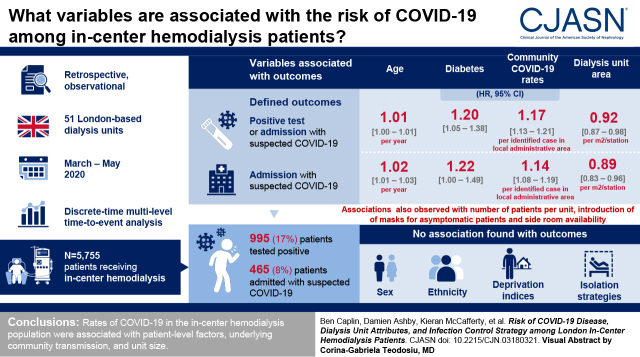
Data on 5755 patients dialyzing in 51 units were analyzed; 990 (17%) tested positive and 465 (8%) were admitted with suspected COVID-19 between March 2 and May 31, 2020. Outcomes were associated with age, diabetes, local community COVID-19 rates, and dialysis unit size. A greater number of available side rooms and the introduction of mask policies for asymptomatic patients were inversely associated with outcomes. No association was seen with sex, ethnicity, or deprivation indices, nor with any of the different isolation strategies.
Conclusions
Rates of COVID-19 in the in-center hemodialysis population relate to individual factors, underlying community transmission, unit size, and layout.
Introduction
Patients with kidney failure and those receiving KRT are among the highest-risk groups for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–related disease and death (1). Alongside kidney dysfunction, this group of patients exhibits a large burden of risk factors for coronavirus disease 2019 (COVID-19), including over-representation of Black and minority ethnic groups and socioeconomic disadvantage, as well as high rates of diabetes and cardiovascular disease. Furthermore, those receiving in-center hemodialysis cannot shield or self-isolate. National data confirm that patients with kidney failure have been disproportionately affected by COVID-19, with 822 of the 36,437 COVID-19–associated deaths reported in England as of July 15, 2020 having occurred in patients with established kidney disease (2, 3). The majority of these have been in patients receiving dialysis treatments, and at least 623 occurred in patients on in-center hemodialysis (2), representing 2% of all COVID-19 deaths in the United Kingdom, despite making up <0.1% of the population. However, rates of infection in London hemodialysis units varied widely, from just a few cases to 30% of patients.
In London, dialysis is organized geographically, with seven tertiary National Health Service (NHS) kidney centers providing local care to patients on the basis of their home address. Each center provides long-term dialysis in units colocated within the main hospital, at another hospital, or in standalone community locations (satellite units), and each unit varies in size (from six to 66 stations).
In line with published guidelines, during the first phase of the pandemic, all dialysis units attempted to identify, isolate, and, where possible, test patients arriving for treatment with symptoms consistent with COVID-19 (4). Nurses caring for the patients wore personal protective equipment as per NHS guidelines (5) only when delivering direct care to patients with known or suspected disease. In addition, kidney centers across London introduced masking policies at different times and used varied patient segregation strategies (for isolation and cohorting of patients with known or suspected disease) to reduce spread of disease. We set out to identify risk factors associated with the burden of disease during the first phase of the COVID-19 pandemic.
Materials and Methods
Study Population
All prevalent London-based patients on in-center hemodialysis on March 2, 2020 were included in the analysis. Patients receiving short-term hemodialysis therapy for AKI were excluded. Patients were assigned to the unit where they dialyzed on March 2nd, except where that unit became a test-positive isolation unit, in which case they were assigned to the first unit they dialyzed in after transfer to an unaffected unit. Cumulative incidence of COVID-19 test positivity and hospital admission rates were collected from March 2 to May 31, 2020. Approximately 23% of our whole population has already formed the basis of a previous report (6). This work was conducted under approval of the St. George’s Research Ethics Committee (reference no. 283130).
Data Sources and Definitions
Pseudoanonymized demographic and clinical data along with COVID-19 test results and hospital admission dates were collected from electronic records. Dialysis unit characteristics and other unit and center data were collected by structured questionnaire (Supplemental Material). Community COVID-19 cases and deprivation indices on the basis of a 37-variable score reflecting aspects of deprivation assessed by middle layer super output area, a geographic hierarchy used to describe small area statistics, were captured from publicly available datasets (3,7). For these variables, we linked the postcode for each patient to the median deprivation index for all output areas within that postcode and the weekly total of all reported COVID-19 cases averaged across all of the output areas within that postcode.
Outcomes
All estimates of infection were subject to limitations during the study period. Testing for SARS-CoV-2 was undertaken using nasopharyngeal swabs followed by RT-PCR performed independently in each of the seven different centers. Availability of swabs was limited during the early phase of the pandemic, so no testing took place in asymptomatic patients and was done variably in those with mild symptoms that would not require hospitalization. Rates of admission with clinically suspected COVID-19 are less likely to be affected by test availability, although rates will vary according to local practice. We, therefore, examined the rates of two outcomes over the 13-week follow-up period: (1) the first of either a positive SARS-CoV-2 test or admission with suspected COVID-19 and (2) admission with suspected COVID-19.
Analyses
As infections occurred at different time points across London for the primary analysis, we defined the time at risk as the time (in weeks) from first positive test at each dialysis unit. We then used a complementary log-log multilevel discrete time survival model (8) to estimate hazard ratios (HRs) associated with individual- and community-level factors, along with dialysis unit physical attributes and policies. Patients were nested within dialysis units, which were in turn nested within kidney centers to account for clustering of outcomes.
Models were developed sequentially on the basis of prespecified candidate factors known or suspected to be associated with outcomes. At each stage, a complete case analysis was performed. We initially examined associations between individual-level demographic and clinical factors (age, sex, ethnicity, diabetes, and deprivation index) and outcomes. All clinical and demographic variables were retained in subsequent models. We then added estimates of time-dependent community transmission (lagged by 0, 1, 2, and 3 weeks), followed by physical attributes of the dialysis units. Variables either that improved fit (as judged by the −2 log likelihood) or where the 95% confidence interval (95% CI) for the HR did not include unity were retained (although for time-lagged variables, only the time lag with the largest effect size was retained). Where two or more correlated explanatory variables were associated with outcome when examined individually (e.g., prevalent patients and number of stations), the variables with the least missing data or that led to the greatest improvement in model fit were retained.
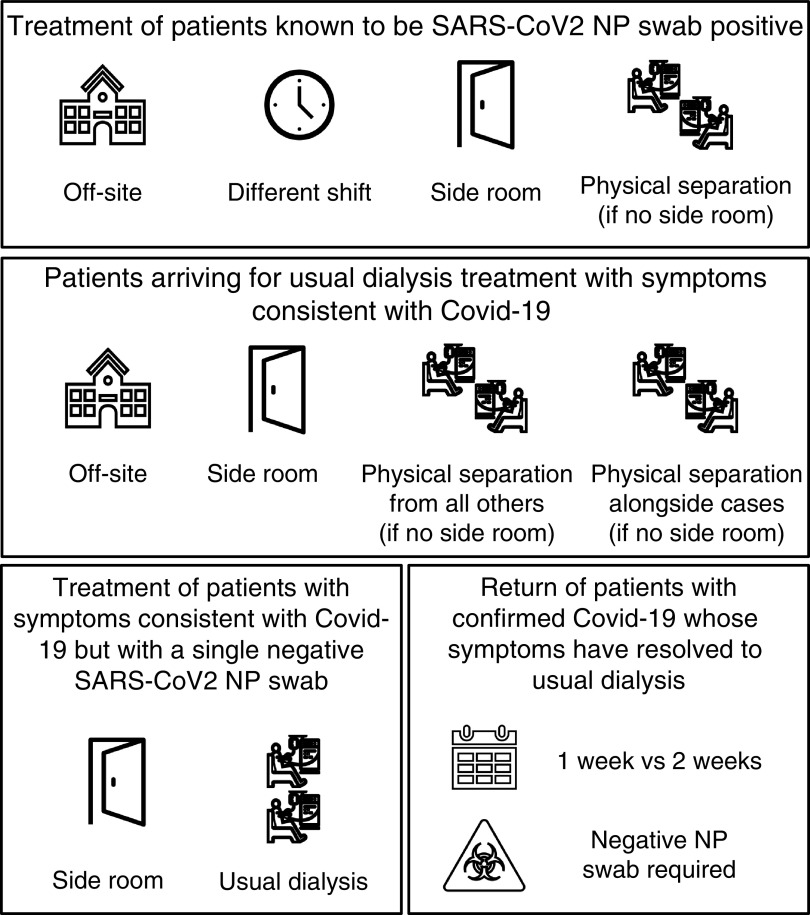
The above then formed the basis to examine individual associations between the various isolation and deisolation strategies, as well as patient and staff masking policies as time-dependent variables (the latter three variables again were lagged by 0, 1, 2, and 3 weeks). For the analysis of isolation strategies, responses were grouped with a view of providing the most useful comparisons as outlined in Supplemental Table 1.
Finally, we presented the coefficients for all of the retained potentially explanatory variables (i.e., not including contemporaneous staff masking) that were associated with the outcome from the unit attributes and masking analyses in a multivariable model.
To understand the relative contribution of each group of variables that was associated with outcome (individual risk factors, community disease, unit attributes, and masking), we examined the area under the receiver operating curve (AUROC) with each model. Finally, we explored the difference in absolute risks associated with the introduction of a policy of asymptomatic patient masking 2 weeks beforehand by estimating the predicted marginal risk with and without the mask policy.
Sensitivity Analyses
We conducted identical models using calendar week follow-up time rather than weeks from the first test-positive case to explore whether the alignment of outbreaks for the analysis had introduced substantial bias. The α was set at 0.05. As this was an exploratory study, no adjustments for multiple testing were made. All analyses were performed in Stata v.16.
Results
Patient Population, Center Policies, and Outcomes
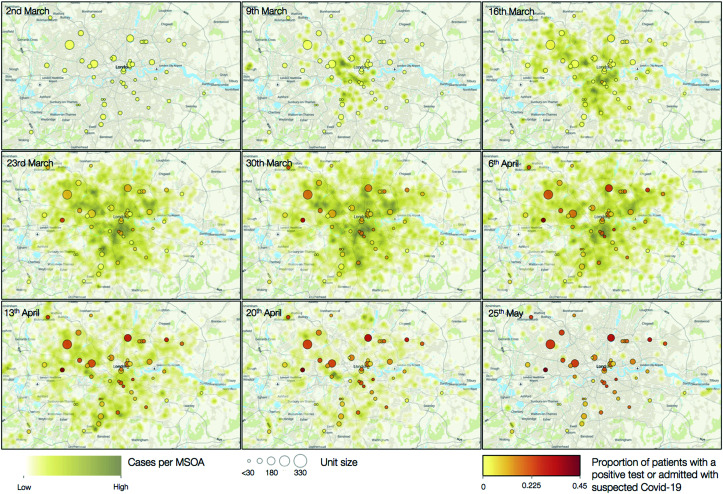
After exclusions (Supplemental Figure 1), we analyzed data on 5755 patients in 51 individual dialysis units from seven kidney centers; 1339 (23%) of these were previously reported (6). Mean age was 63 years (SD of 15), 2229 (39%) were women, and 2401 (42%) had a diagnosis of diabetes (Table 1). There was a broad range of ethnicities represented among the patient population, with 1261 (22%) Asian (predominantly South Asian), 1684 (29%) Black, and 2003 (35%) White. Units dialyzed between eight and 338 patients (Table 2, Supplemental Figure 2). Isolation strategies by center are illustrated in Figure 1 (with further detail in Supplemental Table 2). Introduction of mask-wearing policies occurred over the first 7 weeks. Over the course of the 13-week follow-up, the outcome of test positivity or hospital admission occurred in 990 patients (17%) (Figure 2), and almost half of these, 465 (8%), met the admission outcome. However, these figures encompassed substantial between-unit variation, with first cases occurring between week 1 and week 6 and between 0% and 44% of patients reaching the outcome of test positivity or admission (Figure 3). Across all centers, following the first swab-positive case, the HR peaked in the third week for the outcome of test positivity or admission (HR, 2.1; 95% CI, 1.9 to 2.4 versus week 1) (Supplemental Figure 3) and the fourth week for admission alone (HR, 2.8; 95% CI, 2.3 to 3.3 versus week 1) (Supplemental Figure 3). When examining both outcomes by calendar date, estimates were less precise, but the peak for both outcomes occurred in the fifth week of follow-up.
Table 1.
Patient demographics, outcomes, and community cases
| Characteristic | Center | |||||||
|---|---|---|---|---|---|---|---|---|
| A, n=742 | B, n=645 | C, n=325 | D, n=1179 | E, n=1339 | F, n=840 | G, n=685 | Total, n=5755 | |
| Patients | ||||||||
| Sex, men | 460 (62%) | 405 (63%) | 208 (64%) | 717 (64%) | 794 (59%) | 535 (64%) | 407 (59%) | 3526 (61%) |
| Ethnicity | ||||||||
| Asian | 101 (14%) | 50 (8%) | 83 (25%) | 353 (30%) | 582 (43%) | 65 (8%) | 27 (4%) | 1261 (22%) |
| Black | 200 (27%) | 274 (42%) | 109 (33%) | 335 (28%) | 356 (27%) | 119 (14%) | 291 (42%) | 1684 (29%) |
| White | 252 (34%) | 245 (38%) | 83 (25%) | 287 (24%) | 386 (29%) | 497 (59%) | 253 (37%) | 2003 (35%) |
| Other | 189 (25%) | 76 (12%) | 50 (15%) | 181 (15%) | 15 (1%) | 159 (19%) | 114 (17%) | 784 (14%) |
| Age, yr, mean (SD) | 64 (15) | 62 (15) | 64 (16) | 61 (15) | 65 (14) | 66 (15) | 62 (15) | 63 (15) |
| Diabetes | 370 (50%) | 261 (40%) | 129 (40%) | 394 (33%) | 619 (46%) | 351 (42%) | 277 (40%) | 2401 (42%) |
| Postcode median deprivation index rank, mean (SD) | 13,280 (6621) | 12,442 (6254) | 19,172 (6245) | 10,519 (5160) | 13,497 (5639) | 19,978 (7541) | 13,055 (6564) | 14,041 (6873) |
| Outcomes | ||||||||
| Test positive or admission | 168 (23%) | 101 (16%) | 27 (8%) | 208 (18%) | 305 (23%) | 84 (10%) | 97 (14%) | 990 (17%) |
| Admission | 105 (14%) | 40 (6%) | 18 (5%) | 68 (6%) | 148 (11%) | 50 (6%) | 36 (5%) | 465 (8%) |
| Community cases, cases/middle layer super output area, mean (SD) | ||||||||
| Week 1 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Week 2 | 0.3 (0.5) | 0.3 (0.4) | 0.6 (0.5) | 0.1 (0.2) | 0.4 (0.5) | 0.2 (0.2) | 0.4 (0.5) | 0.3 (0.4) |
| Week 3 | 1.7 (1.1) | 2.1 (1.1) | 2.4 (1.3) | 1.2 (0.7) | 2.4 (1.2) | 1.2 (1.1) | 2.0 (1.4) | 1.8 (1.3) |
| Week 4 | 4.6 (1.9) | 4.8 (2.0) | 5.2 (1.4) | 4.0 (1.4) | 5.5 (2.2) | 3.3 (2.2) | 4.7 (2.3) | 4.6 (2.1) |
| Week 5 | 5.6 (2.2) | 7.5 (1.5) | 6.5 (2.0) | 6.5 (1.2) | 6.6 (2.7) | 5.3 (2.9) | 6.5 (2.5) | 6.3 (2.4) |
| Week 6 | 4.3 (1.6) | 6.0 (1.5) | 5.0 (1.4) | 5.6 (1.5) | 5.2 (1.8) | 5.0 (2.0) | 5.1 (1.8) | 5.2 (1.8) |
| Week 7 | 2.9 (1.1) | 3.8 (1.0) | 3.6 (1.7) | 2.9 (1.2) | 4.5 (1.6) | 5.0 (1.7) | 3.1 (1.1) | 3.8 (1.6) |
| Week 8 | 2.5 (1.7) | 2.5 (0.8) | 2.0 (1.3) | 2.0 (1.0) | 3.3 (1.4) | 3.8 (1.6) | 2.1 (1.0) | 2.7 (1.5) |
| Week 9 | 1.4 (0.9) | 2.2 (0.9) | 2.0 (1.1) | 1.7 (1.1) | 2.6 (1.3) | 3.1 (1.4) | 1.8 (1.2) | 2.2 (1.3) |
| Week 10 | 0.6 (0.7) | 1.0 (0.5) | 0.6 (0.6) | 1.1 (0.9) | 1.2 (0.9) | 1.3 (0.9) | 0.8 (0.7) | 1.0 (0.9) |
| Week 11 | 0.3 (0.5) | 0.8 (0.4) | 0.6 (0.6) | 0.2 (0.4) | 0.8 (1.0) | 1.0 (0.9) | 0.6 (0.6) | 0.6 (0.8) |
| Week 12 | 0.2 (0.3) | 0.7 (0.8) | 0.3 (0.3) | 0.2 (0.2) | 0.4 (0.4) | 0.7 (0.8) | 0.4 (0.9) | 0.4 (0.6) |
| Week 13 | 0.1 (0.2) | 0.2 (0.7) | 0.1 (0.2) | 0.1 (0.2) | 0.2 (0.2) | 0.3 (0.4) | 0.4 (1.0) | 0.2 (0.5) |
Values are N (%) unless otherwise stated.
Table 2.
Unit-level characteristics
| Characteristic | Center | |||||||
|---|---|---|---|---|---|---|---|---|
| A, n=5 | B, n=7 | C, n=5 | D, n=11 | E, n=8 | F, n=8 | G n=7 | Total, n=51 | |
| Unit characteristics | ||||||||
| Prevalent patients, median (IQR) | 189 (76–172) | 80 (62.5–92.5) | 82 (34–82) | 123 (47–132.5) | 144 (116–206) | 130 (75.5–131) | 103 (67–105) | 100 (70–132) |
| Stations, median (IQR) | 29 (16–30) | 17 (12–20) | 18 (6–18) | 20 (18–27) | 24.5 (23–45) | 24 (19.5–25.5) | 20 (20–28) | 20 (18–26) |
| Side rooms,a n/20 stations, median (IQR) | 1.7 (1.7–2.5) | 3.3 (0–4.0) | 2.7 (2.2–6.7) | 0.0 (0.0–1.0) | 0.0 (0.0–0.3) | 3.3 (3.1–4.6) | 3.0 (2.1–5.0) | 2.2 (0.0–3.3) |
| Waiting room,b m2 per station, median (IQR) | 1.0 (0.7–1.3) | 1.25 (0.7–1.7) | 1.6 (0.5–4.0) | 0.9 (0.8–1.0) | 1.4 (1.1–1.7) | 1.1 (0.4–1.6) | — | 1.30 (0.4–4.0) |
| Dialysis area,b m2 per station, median (IQR) | 7.7 — | 10.3 (9.0–10.8) | 12.9 (9.2–14.6) | 13.6 (11.9–14.9) | 11.2 (10.2–14.4) | 12.1 (10.3–12.3) | — | 11.2 (9.6–12.9) |
| Station distance,c mean (SD) | 2.49 (0.69) | 2.14 (0.37) | 2.39 (0.58) | 2.68 (0.77) | 2.21 (0.41) | 2.07 (0.45) | — | 2.39 (0.59) |
| Masking | ||||||||
| Week (or range) of introduction of staff masks for all | 4 | 6 | (1–6) | 3 | 4 | (1–6) | (2–6) | (1–6) |
| Week (or range) of introduction of patient masks for all | 5 | 6 | (2–6) | 3 | 7 | (1–6) | (2–4) | (1–7) |
Values are N (%) unless otherwise stated. IQR, interquartile range; —, not available.
Data are available for 46 units.
Data are available for 31 units.
Data are available for 33 units.
Figure 1.
Isolation and deisolation strategies for management of patients with clinically suspected or confirmed coronavirus disease 2019 (COVID-19). NP, nasopharyngeal; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 2.
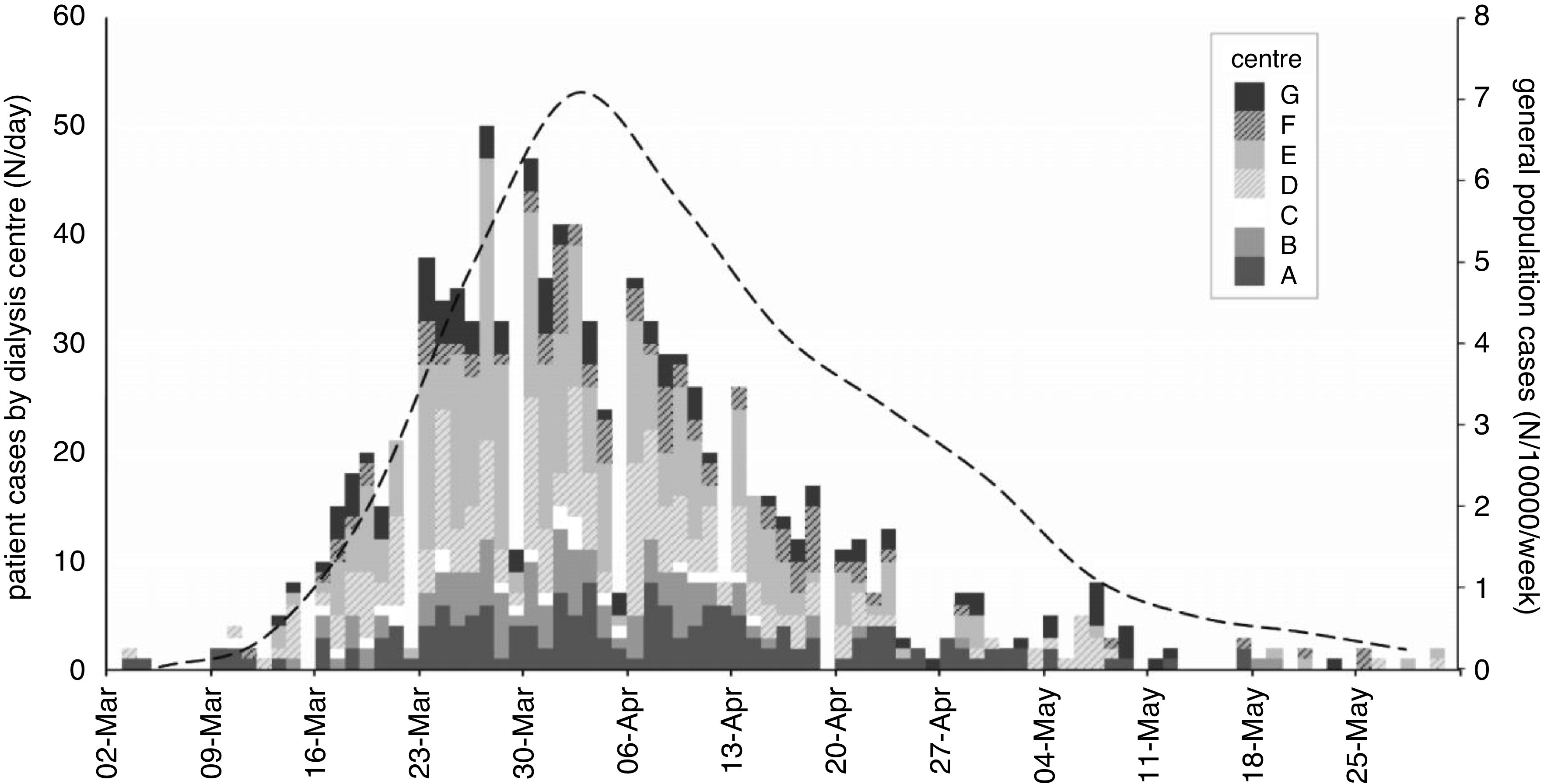
Epidemic timeline in patients on in-center hemodialysis across London. Patient cases (first of positive test or admission) by renal center expressed as actual numbers per day. Cases in the general population are available weekly by location and calculated by postcode per 10,000 population. The whole population average (dashed line) is weighted by the number of patients per postcode.
Figure 3.
Geographic distribution of cases by dialysis unit by week for the first 8 weeks of the pandemic and at the final follow-up week. Each in-center hemodialysis unit is represented by a circle. Circle size indicates the number of patients dialyzed. Circle color indicates the cumulative proportion of patients who tested positive for COVID-19 or were admitted with suspected COVID-19. Underlying green intensity reflects the number of cases in each middle layer super output area (not necessarily reflective of patients’ home locality as most, but not all, patients dialyze in their closest unit). Three units are not represented as they fall outside of the area of the map. Dates represent the first day of the week represented. MSOA, middle layer super output area.
Clinical and Demographic Variables and Estimates of Disease in Patients’ Local Communities
As expected, in the clinical and demographic model for both time to test positivity or admission and admission alone, age (HR, 1.01; 95% CI, 1.00 to 1.01 and HR, 1.02; 95% CI, 1.01 to 1.03 per year, respectively) and presence of diabetes (HR, 1.20; 95% CI, 1.05 to 1.38 and HR, 1.22; 95% CI, 1.00 to 1.49, respectively) were associated with outcomes (Table 3). There was also a strong association with estimates of disease in patients’ local community, most strongly in the concurrent week (HR, 1.17; 95% CI, 1.13 to 1.21 and HR, 1.14; 95% CI, 1.08 to 1.19 per case per middle layer super output area in patients’ home postcode, respectively) (Table 3).
Table 3.
Individual associations with study outcomes
| Characteristic | Positive Test or Admission | Admission | ||
|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval | |
| Clinical and demographic factors, mutually adjusted | ||||
| Age, per yr | 1.01 | 1.00 to 1.01 | 1.02 | 1.01 to 1.03 |
| Men | 0.94 | 0.82 to 1.07 | 1.04 | 0.85 to 1.26 |
| Ethnicity | ||||
| Asian | 1.12 | 0.93 to 1.36 | 1.05 | 0.79 to 1.38 |
| Black | 1.11 | 0.93 to 1.33 | 1.16 | 0.90 to 1.51 |
| Other | 0.90 | 0.71 to 1.13 | 0.75 | 0.52 to 1.08 |
| Diabetes | 1.20 | 1.05 to 1.38 | 1.22 | 1.00 to 1.49 |
| Median IMD rank, per 100 | 1.00 | 1.00 to 1.00 | 1.00 | 1.00 to 1.00 |
| Community case burden adjusted for all clinical and demographic factors, cases per output area in home postcode | ||||
| Community cases, week 0 | 1.17 | 1.13 to 1.21 | 1.14 | 1.08 to 1.19 |
| Community cases, week −1 | 1.11 | 1.06 to 1.14 | 1.11 | 1.05 to 1.16 |
| Community cases, week −2 | 1.01 | 0.97 to 1.05 | 1.05 | 0.99 to 1.11 |
| Community cases, week −3 | 0.90 | 0.85 to 0.94 | 0.96 | 0.89 to 1.02 |
| Unit attributes individually adjusted for all clinical and demographic factors and community disease burden | ||||
| Prevalent patients, n | 1.00 | 1.00 to 1.01 | 1.00 | 1.00 to 1.01 |
| Stations,a n | 1.01 | 1.00 to 1.03 | 1.02 | 1.01 to 1.04 |
| Dialysis area,b m2 per station | 0.92 | 0.87 to 0.98 | 0.89 | 0.83 to 0.96 |
| Waiting room size,c m2 per station | 1.02 | 0.99 to 1.05 | 1.01 | 0.99 to 1.04 |
| Station distance,d m2 | 0.68 | 0.49 to 0.94 | 1.03 | 0.67 to 1.59 |
| Side rooms,e n/20 stations | 0.88 | 0.82 to 0.94 | 0.90 | 0.80 to 1.01 |
| Isolation policies individually adjusted for clinical and demographic factors, community disease burden, unit prevalent patients, and side rooms a | ||||
| Isolation of test-positive patients off site | 0.88 | 0.68 to 1.13 | 0.78 | 0.53 to 1.16 |
| Isolation of symptomatic arrivals off site or in SR | 1.10 | 0.76 to 1.57 | 0.79 | 0.42 to 1.49 |
| Return of test-negative patients to usual dialysis | 0.89 | 0.65 to 1.20 | 0.89 | 0.48 to 1.65 |
| Return of test-positive patients after 2 wk (versus 1 wk) | 0.87 | 0.65 to 1.15 | 0.64 | 0.39 to 1.06 |
| Return of test-positive patients after testing negative | 0.96 | 0.72 to 1.29 | 0.77 | 0.47 to 1.27 |
| Masking policies individually adjusted for clinical and demographic factors, community disease burden, unit prevalent patients, and side rooms a | ||||
| Staff masks, week 0 | 1.47 | 1.12 to 1.92 | 1.71 | 1.10 to 2.65 |
| Staff masks, week −1 | 1.06 | 0.84 to 1.34 | 1.41 | 0.97 to 2.04 |
| Staff masks, week −2 | 1.04 | 0.82 to 1.32 | 0.99 | 0.70 to 1.39 |
| Staff masks, week −3 | 1.12 | 0.86 to 1.46 | 0.95 | 0.65 to 1.38 |
| Patient masks, week 0 | 1.07 | 0.86 to 1.32 | 1.03 | 0.75 to 1.41 |
| Patient masks, week −1 | 0.92 | 0.73 to 1.15 | 0.84 | 0.60 to 1.17 |
| Patient masks, week −2 | 0.87 | 0.66 to 1.15 | 0.64 | 0.44 to 0.93 |
| Patient masks, week −3 | 0.89 | 0.68 to 1.16 | 0.69 | 0.45 to 1.04 |
P < 0.05 for coefficients in bold. Data are available on n=5316 unless stated. IMD, Index of Multiple Deprivation; SR, side room.
n=5217.
n=3730.
n=3536.
n=4436.
n=4928.
Dialysis Unit Characteristics
After adjustment for individual-level variables and community cases, a number of dialysis unit characteristics were associated with the risk of the outcomes, specifically unit size and layout (Table 3). These included number of dialysis stations (HR, 1.04; 95% CI, 1.00 to 1.28 and HR, 1.02; 95% CI, 1.01 to 1.03 per station for test positivity or admission and admission alone, respectively) and dialysis unit area per station (HR, 0.92; 95% CI, 0.87 to 0.98 and HR, 0.89; 95% CI, 0.83 to 0.96 per m2 for test positivity or admission and admission alone, respectively) as well as distance between dialysis stations and number of side (isolation) rooms per station for the test positivity outcome but not the admission alone outcome. This was also the case for both outcomes when examining the association with number of prevalent patients. Many of these characteristics were strongly correlated (Supplemental Figure 4) (e.g., smaller units with fewer stations typically had relatively more side rooms; therefore, only the number of prevalent patients and number of side rooms per station were retained for the modeling of infection control strategies, masking, and staff illness).
Isolation Strategies and Mask Policy
When examining the various units’ isolation strategies (after adjustment for clinical and demographic factors, community cases, and physical unit characteristics), we found no evidence for a difference in either outcome with any of (1) treating all known positive patients on a separate site; (2) the different isolation approaches applied to newly suspected cases attending the dialysis unit; or (3) deisolation policies, either for those who had tested positive (by time or requirement for a negative swab) or symptomatic patients who had tested negative (Table 3). We observed a reduction in admissions (but not test-positive or admission outcome) following institution of patient masking 2 weeks previously (HR, 0.64; 95% CI, 0.44 to 0.93). No similar effect was seen for staff masking in our primary analyses; rather, there was a positive association with both outcomes with institution of staff masking in the concurrent week (HR, 1.47; 95% CI, 1.12 to 1.92 and HR, 1.71; 95% CI, 1.10 to 2.65 for test positivity or admission and admission alone, respectively).
The final multivariable model (Table 4) demonstrated age, diabetes, number of patients in the unit, and estimates of community transmission for the current week as positively associated with both outcomes. Number of side rooms (for the test-positive outcome only) and introduction of patient masking 2 weeks previously (for admission only) were inversely associated with outcomes. Sensitivity analyses using calendar week rather than time from first case demonstrated broadly consistent findings, although a number of coefficients differed and 95% CIs were generally wider for time-dependent effects (Supplemental Table 2). The AUROC analysis (Supplemental Table 3) suggested that community transmission explained a substantial proportion of the variation in the model for the test-positive or admission outcome, whereas individual factors were more important for the admission outcome. The modeled absolute risk reduction associated with a policy introduction of asymptomatic patient masking 2 weeks previously was in the range 0.3%–0.4% during the weeks when most admissions occurred (Supplemental Table 4), although the proportion of the follow-up period when most centers practiced different patient masking policies was limited to just a few weeks.
Table 4.
Multivariable coefficients
| Charateristic | Positive Test or Admission | Admission | ||
|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval | |
| Age, per yr | 1.01 | 1.00 to 1.01 | 1.02 | 1.01 to 1.03 |
| Men | 0.95 | 0.83 to 1.09 | 1.03 | 0.84 to 1.41 |
| Ethnicity | ||||
| Asian | 1.06 | 0.88 to 1.29 | 1.00 | 0.75 to 1.33 |
| Black | 1.08 | 0.90 to 1.30 | 1.12 | 0.86 to 1.45 |
| Other | 0.88 | 0.70 to 1.13 | 0.71 | 0.49 to 1.04 |
| Diabetes | 1.16 | 1.04 to 1.41 | 1.21 | 0.99 to 1.48 |
| Median deprivation index rank, per 100 | 1.00 | 1.00 to 1.00 | 1.00 | 0.98 to 1.02 |
| Community cases, week 0, case per output area in home postcode | 1.15 | 1.12 to 1.19 | 1.15 | 1.09 to 1.22 |
| Side rooms, n/20 stations | 0.88 | 0.83 to 0.95 | 0.88 | 0.77 to 1.01 |
| Prevalent patients, n | 1.00 | 1.00 to 1.01 | 1.00 | 1.00 to 1.01 |
| Patient masks, week −2 | 0.82 | 0.64 to 1.04 | 0.64 | 0.44 to 0.93 |
P=0.05 for coefficients in bold. Mutually adjusted. n=4928
Discussion
We describe the individual and community factors, dialysis unit, masking policies, and isolation strategies associated with risk of COVID-19 in London patients on in-center hemodialysis during the first phase of the pandemic between March and May 2020. To our knowledge, aside from registry data, which lacks this degree of granularity (9), this report encompasses the largest number of cases of COVID-19 in hospital-based patients on dialysis and is the first to examine the evolution of the disease in individual dialysis units over time. This analysis provides important insights into the role of endemic disease, unit characteristics, mask policies, and isolation strategies on COVID-19 transmission in patients on hemodialysis. Our data confirm that patients on dialysis are at high risk of symptomatic illness and hospital admission from COVID-19, with almost 20% of the patients on prevalent dialysis reaching the primary outcome of test positivity or admission. This proportion is similar to those reported from other European and UK centers (6, 10). However, it is substantially higher than those reported in France (9, 11) and China (12), albeit over shorter time frames. Consistent with the published data on patients without kidney disease (13) and patients with kidney failure (9), both age and diabetes were strong risk factors for outcomes. Unlike other studies, we did not observe associations with ethnicity (14) or measures of deprivation. However, unlike others, we adjusted for community burden of disease, and these negative findings were apparent even in those models adjusted solely for individual-level characteristics. Therefore, it is possible that the COVID-19 disease risk associated with dialysis treatment overwhelms the effect of these social/demographic factors.
The importance of local community COVID-19 disease as reflected in the time-dependent rates of disease near patients’ homes is highlighted in this study. The AUROC analysis suggests that community disease burden explains a substantial proportion of the variability in test positivity in this cohort, although only those in the general population with disease severity requiring hospitalization were being tested for COVID-19 during this period, making it difficult to draw specific conclusions about the level of community transmission that associates with a particular risk to patients on dialysis. These findings suggest that disease in dialysis units is not occurring as isolated “outbreaks” within treatment centers, but rather is dependent on introductions from contacts, in patients’ homes, and from local communities. Consistent with this is the observation that larger units suffered a higher burden of disease, with more potential direct and indirect contacts between patients and others in the community.
Nonetheless, a number of (inter-related) dialysis unit characteristics were also associated with the outcomes, suggesting that within-unit spread is also a factor. The number of side rooms per dialysis station was strongly associated with both outcomes in all models and suggests that the capacity to isolate patients with suspected or confirmed disease is protective against onward transmission. Similarly, measures of distancing between patients, estimated by total dialysis area or between-station distance, were both inversely associated with outcomes (although not independent of other unit factors). Consistent with the importance of asymptomatic transmission, the introduction of a mask policy for all patients was associated with reduced risk of admission. Although specific to this study population and not generalizable, the modeled estimates suggested that introducing a policy of asymptomatic patient mask wearing was associated with a small absolute reduction in probability of admission. Furthermore, although overall a small effect (at least partly because the introduction of mask policy varied only a few weeks between centers), this association was observed after a 2-week lag, consistent with the reported time from infection to hospitalization. The lack of a similar association with the test-positivity outcome may reflect lack of power or an alternative biologic explanation. Taken together, the above findings suggest that transmission from those without clinically suspected disease is an important factor in determining COVID-19 burden in dialysis units (15).
Importantly, none of the varying isolation strategies used by the different kidney centers were associated with outcomes. These policies were instituted in addition to universal precautions mandated for the care of symptomatic patients across all centers, so the power to detect what may be small additional protective effects is likely to be low. These data suggest that community levels of COVID-19, along with a small contribution of unit factors and masking, determine how the infection burden evolves. The relevance of these data for planning for future waves of disease may be modified by (1) unrestricted/asymptomatic testing that is now available, (2) the use of surgical face masks by asymptomatic patients and those treating them, and (3) the potential effect of vaccination or acquired immunity in the population.
This work has a number of limitations, including the observational design, which restricts the conclusions that can be drawn about causality. Data were collected retrospectively, and many of the dialysis unit–level and kidney center–level factors were on the basis of questionnaires, which could have been interpreted differently between centers, despite the validation checks performed. We used a discrete time survival approach and, therefore, cannot account for the effect of recovery or reinfection on disease transmission, but given the short time frame and, presumably, an initially fully susceptible population, this is unlikely to affect our conclusions. Although we used two different outcome measures with broadly similar results, we will have missed large numbers of asymptomatic cases with the test-positive outcome, as serologic studies confirm that large numbers of patients in London dialysis units seroconverted who were either never tested or tested negative during the early phase of the pandemic (16).
In this comprehensive study of COVID-19 disease in the dialysis units across London between March and May 2020, we found that individual factors and local community case rates along with dialysis unit size and layout associate with the risk of disease. Masking of asymptomatic patients was associated with a reduction in admissions, supporting the concept that transmission from asymptomatic patients is important, but no differences were seen between the different infection control strategies aimed at managing those with confirmed or clinically suspected disease. This work confirms the large burden of COVID-19 experienced by the dialysis population and highlights the need to prioritize this vulnerable group in strategies aimed at reducing spread, protecting individuals, and/or treatment of established disease.
Disclosures
M. Antonelou reports receiving research funding from Medical Research Council. D. Ashby reports receiving honoraria from Fibrogen. D. Banerjee reports receiving research funding from the British Heart Foundation; receiving grants from AstraZeneca and Kidney Research UK; and receiving honoraria from AstraZeneca, Pfizer, and Viforpharma. S.A. Blakey reports employment with West London Renal and Transplant Centre. K. Bramham reports consultancy agreements with Alexion; receiving honoraria from Alexion and Otsuka; and serving as a scientific advisor or member of Alexion. B. Caplin reports consultancy agreements with LifeArc and receiving research funding from AstraZeneca and grants from Colt Foundation, Medical Research Council, and Royal Free Charity outside the submitted work. R. Corbett has been issued Patent WO2017148836A1: “A device for maintaining vascular connections.” M.L. Ford reports other interests/relationships with the AstraZeneca advisory board, which led to the publication of the paper. A. Frankel reports receiving research funding from Boehringer Ingelheim/Lilly Alliance and receiving honoraria from AstraZeneca, Boehringer Ingelheim/Lilly Alliance, Merck Sharp & Dohme, Napp Pharmaceuticals Limited, and Novo Nordisk. R. Hull reports consultancy agreements with AstraZeneca, Pharmocosmos UK Ltd., and Travere Pharmaceuticals; speakers bureau for Napp Phamaceuticals; serving as an elected council member of Renal Association, UK; and other interests/relationships with Joint Specialist Committee Renal Medicine, Royal College Physicians, London. K. McCafferty reports receiving research funding from AstraZeneca and receiving honoraria from Bayer, Napp, Pharmacosmos, and Vifor Fresenius. A.D. Salama reports receiving research funding from Chiesi and Natera; receiving honoraria from AnaptysBio, AstraZeneca, Hansa Medical, and Vifor Pharmaceuticals; and serving as Nephrology Dialysis Transplantation Editor and a UK Renal Association Executive Member. C.C. Sharpe reports consultancy agreements with Novartis Pharmaceuticals; receiving honoraria from Napp Pharmaceuticals; serving as an Editor for BMC Nephrology and as a trustee and treasurer for the Renal Association; and speakers bureau for Napp Pharmaceuticals. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The authors acknowledge the key role of the clinical nursing and medical staff who also contributed to the Pan London Covid Renal Audit.
D. Ashby, D. Banerjee, K. Bramham, B. Caplin, A. Frankel, E. Lioudaki, D. Makanjuola, K. McCafferty, A.D. Salama, and C.C. Sharpe designed the study; D. Ashby and B. Caplin analyzed the data; M. Antonelou, E. Asgari, D. Banerjee, S.A. Blakey, D.C.B. Braide-Azikwe, K. Bramham, G. Clark, N. Cole, R. Corbett, H. Cronin, M.L. Ford, N.J. Hayes, N. Kumar, D. Makanjuola, B. Manson, K. McCafferty, T. Roper, A.D. Salama, A. Sarnowski, and V. Srinivasa collected the raw data; and all authors approved the final manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Contributor Information
Collaborators: The Pan-London COVID-19 Renal Audit Group authors are Marilina Antonelou, Asgari Elham, Ashby Damien, Banerjee Debasish, Blakey Sarah, Bramham Kate, Brewster Rosalind, Briade-Azikwe Dandisonba, Cairns Hugh, Caplin Ben, Chowdhury Paramit, Clark Grace, Cole Nicholas, Corbett Richard, Cove-Smith Andrea, Cronin Helen, Davari Maria, Dias Abigail, Evans Kevin, Forbes Suzanne, Ford Martin, Frankel Andrew, Gnansampanthan Sahana, Goodlad Catriona, Hayes Nathan, Hendra Heidy, Hull Richard, Kumar Nicola, Lioudaki Eirini, Loucaidou Marina, Makanjuola David, Manson Bethia, McCafferty Kieran, McGuinness Daniel, Neradova Aegida, Phanish Mysore, Price Katherine, Rajakariar Ravi, Rankin Alex, Roper Tayeba, Roth Noam, Salama Alan, Sarnowski Alexander, Schott Cassim, Sharpe Claire, Srinivasa Vinay, Tandaric Damir, Thyagarajan Sujanita, Vajgel Gisele, and Young Gregor
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03180321/-/DCSupplemental.
Supplemental Table 1. Isolation and deisolation strategies for management of patients with clinically suspected or confirmed COVID-19 by center.
Supplemental Table 2. Sensitivity analysis—models using calendar week follow-up.
Supplemental Table 3. ROC curve analysis of models.
Supplemental Table 4. Modeled absolute risks of admission associated with a policy of asymptomatic patient masking 2 weeks following introduction by week.
Supplemental Figure 1. Study flow chart.
Supplemental Figure 2. In-center hemodialysis units dialyzing test-negative patients in London during the COVID-19 pandemic colored by responsible renal center.
Supplemental Figure 3. Hazard ratios by week using the two different follow-up scales.
Supplemental Figure 4. Ethnicity distribution and associations between unit-level characteristics.
Supplemental Material. Data collection tool and STROBE checklist.
References
- 1.Valeri AM, Robbins-Juarez SY, Stevens JS, Ahn W, Rao MK, Radhakrishnan J, Gharavi AG, Mohan S, Husain SA: Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol 31: 1409–1415, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UK Renal Registry: COVID-19 surveillance report for renal centres in the UK: All regions and centres. Bristol, United Kingdom, UK Renal Registry, 2020
- 3.Department of Health: Coronavirus (COVID-19) in the UK, 2020. Available at: https://coronavirus.data.gov.uk. Accessed June 16, 2020
- 4.Basile C, Combe C, Pizzarelli F, Covic A, Davenport A, Kanbay M, Kirmizis D, Schneditz D, van der Sande F, Mitra S: Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant 35: 737–741, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Health: Guidance on infection prevention and control for COVID-19, 2020. Available at: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control#history. Accessed July 1, 2020
- 6.Corbett RW, Blakey S, Nitsch D, Loucaidou M, McLean A, Duncan N, Ashby DR; West London Renal and Transplant Centre: Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol 31: 1815–1823, 2020 [DOI] [PMC free article] [PubMed]
- 7.Office for National Statistics: Index of Multiple Deprivation (December 2019) Lookup in England, 2019. Available at: https://geoportal.statistics.gov.uk/datasets/index-of-multiple-deprivation-december-2019-lookup-in-england. Accessed June 16, 2020
- 8.Austin PC: A tutorial on multilevel survival analysis: Methods, models and applications. Int Stat Rev 85: 185–203, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couchoud C, Bayer F, Ayav C, Béchade C, Brunet P, Chantrel F, Frimat L, Galland R, Hourmant M, Laurain E, Lobbedez T, Mercadal L, Moranne O; French REIN registry: Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int 98: 1519–1529, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, Maffei C, Possenti S, Lucca B, Cortinovis R, Terlizzi V, Zappa M, Saccà C, Pezzini E, Calcaterra E, Piarulli P, Guerini A, Boni F, Gallico A, Mucchetti A, Affatato S, Bove S, Bracchi M, Costantino EM, Zubani R, Camerini C, Gaggia P, Movilli E, Bossini N, Gaggiotti M, Scolari F: A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int 98: 20–26, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller N, Chantrel F, Krummel T, Bazin-Kara D, Faller AL, Muller C, Nussbaumer T, Ismer M, Benmoussa A, Brahim-Bouna M, Beier S, Perrin P, Hannedouche T: Impact of first-wave COronaVIrus disease 2019 infection in patients on haemoDIALysis in Alsace: The observational COVIDIAL study. Nephrol Dial Transplant 35: 1338–1411, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong F, Tang H, Liu L, Tu C, Tian JB, Lei CT, Liu J, Dong JW, Chen WL, Wang XH, Luo D, Shi M, Miao XP, Zhang C: Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol 31: 1387–1397, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B: Factors associated with COVID-19-related death using OpenSAFELY. Nature 584: 430–436, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioannou GN, Locke E, Green P, Berry K, O’Hare AM, Shah JA, Crothers K, Eastment MC, Dominitz JA, Fan VS: Risk factors for hospitalization, mechanical ventilation, or death among 10,131 US veterans with SARS-CoV-2 infection. JAMA Netw Open 3: e2022310, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones NR, Qureshi ZU, Temple RJ, Larwood JPJ, Greenhalgh T, Bourouiba L: Two metres or one: What is the evidence for physical distancing in COVID-19? BMJ 370: m3223, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Clarke C, Prendecki M, Dhutia A, Ali MA, Sajjad H, Shivakumar O, Lightstone L, Kelleher P, Pickering MC, Thomas D, Charif R, Griffith M, McAdoo SP, Willicombe M: High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol 31: 1969–1975, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.