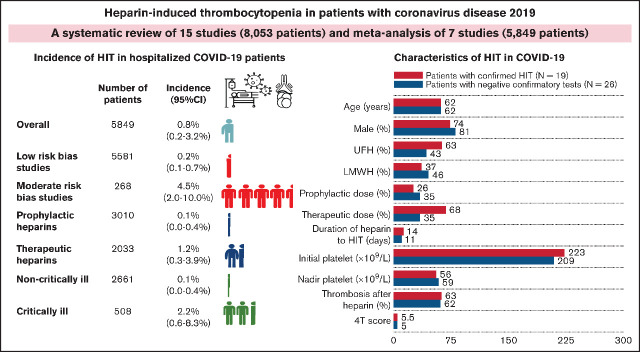
Visual Abstract
Abstract
Heparin thromboprophylaxis is routinely administered during hospitalization for COVID-19. Because of the immune stimulation related to COVID-19, there is ongoing concern regarding a heightened incidence of heparin-induced thrombocytopenia (HIT). We performed a literature search using PubMed, EMBASE, Cochrane, and medRxiv database to identify studies that reported clinical and laboratory characteristics and/or the incidence of HIT in patients with COVID-19. The primary aim was to systematically review the clinical features and outcomes of patients with COVID-19 with confirmed HIT. The secondary objective was to perform a meta-analysis to estimate the incidence of HIT in hospitalized patients with COVID-19. A meta-analysis of 7 studies including 5849 patients revealed the pooled incidence of HIT in COVID-19 of 0.8% (95% confidence interval [CI], 0.2%-3.2%; I2 = 89%). The estimated incidences were 1.2% (95% CI, 0.3%-3.9%; I2 = 65%) vs 0.1% (95% CI, 0.0%-0.4%; I2 = 0%) in therapeutic vs prophylactic heparin subgroups, respectively. The pooled incidences of HIT were higher in critically ill patients with COVID-19 (2.2%; 95% CI, 0.6%-8.3%; I2 = 72.5%) compared with noncritically ill patients (0.1%; 95% CI, 0.0%-0.4%: I2 = 0%). There were 19 cases of confirmed HIT and 1 with autoimmune HIT for clinical and laboratory characterization. The median time from heparin initiation to HIT diagnosis was 13.5 days (interquartile range, 10.75-16.25 days). Twelve (63%) developed thromboembolism after heparin therapy. In conclusion, the incidence of HIT in patients with COVID-19 was comparable to patients without COVID-19, with higher incidences with therapeutic anticoagulation and in critically ill patients.
Introduction
Since the first emerging cluster of pneumonia in China in December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 170 million individuals and caused nearly 4 million deaths worldwide.1 Abnormal coagulation parameters, especially elevated D-dimer levels, were rapidly recognized as the key features of patients infected with SARS-CoV-2 and were associated with poor outcomes, suggesting that hypercoagulation may play roles in the disease pathogenesis.2-5 The early report from China suggested a potential survival benefit of anticoagulation in patients with COVID-19.6
Shortly after its spread to Europe, there were several cohorts that identified the high incidence of thromboembolism in hospitalized patients with COVID-19.7-9 Therefore, several international guidelines recommended anticoagulants for the management of COVID-19–associated coagulopathy and routine pharmacologic thromboprophylaxis for all hospitalized patients with COVID-19 without contraindications.10-13 However, a significant proportion of patients, especially in the intensive care unit (ICU), developed both arterial and venous thromboembolism despite standard-dose thromboprophylaxis.7,14,15 Consequently, many medical centers have implemented intermediate-dose or therapeutic-dose anticoagulants to prevent thromboembolic complications.16 Currently, there are several randomized controlled trials evaluating the efficacy and safety of different intensities of thromboprophylaxis in hospitalized patients with COVID-19.17,18 In a recently published INSPIRATION randomized controlled trial, intermediate-dose prophylactic anticoagulation did not provide additional benefits over standard-dose prophylaxis.19
Heparin-induced thrombocytopenia (HIT) is an uncommon but serious immunologic complication from heparin leading to transient thrombocytopenia accompanied by highly prothrombotic state.20 Diagnosis of HIT, especially in critically ill patients, is challenging because there are many alternative causes of thrombocytopenia.21,22 Nonpathologic antiplatelet 4/heparin antibodies (anti-PF4/H Abs) may also be present in this population.20 The diagnosis of HIT requires confirmatory tests that demonstrate platelet activation of anti-PF4/H Abs in the presence of heparin.
According to the guidelines,10-13 unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) are indicated for hospitalized patients with COVID-19. The wide use of heparin may lead to increasing incidence of HIT, complicating patient care by aggravating thrombocytopenia and intensifying thrombotic risks. Awareness and early recognition are critical for proper management (ie, initiation of nonheparin anticoagulants and avoiding platelet transfusion).20,21
To date, the incidence, clinical characteristics, and impacts of HIT on hospitalized patients with COVID-19 remain largely unknown. We conducted a systematic review to characterize clinical manifestations, laboratory profiles, management, and clinical outcomes of HIT and performed a meta-analysis to estimate the incidence of HIT in hospitalized patients with COVID-19.
Methods
The protocol for this review was prespecified and registered in PROSPERO (CRD42021240788). The study was subsequently conducted following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.23 The primary objective of this study was to systematically characterize clinical and laboratory presentations, diagnosis, management, and clinical outcomes of HIT and HIT with thrombosis in hospitalized patients with COVID-19. The meta-analysis of the incidence of HIT, the incidence of anti-PF4/H Abs, and risks associated with HIT development were planned if there were sufficient data for analysis. The prespecified subgroup analyses including types of heparin (UFH vs LMWH), intensities of heparins (prophylactic vs therapeutic), and severity of patients with COVID-19 (critically ill vs non critically ill) would be performed if there were sufficient data.
Data source, search strategy, and study selection
A systematic search of electronic databases was performed using PubMed, EMBASE, Cochrane Library Database, and the preprint server (medRxiv) from inception to 8 March 2021 and was updated on 14 June 2021 to identify studies reporting cases with confirmed HIT using platelet activation assays and/or incidence of HIT in patients with COVID-19. The following search terms were used: heparin, anticoagulant, anticoagulation, antithrombotic, thrombocytopenia, platelet, platelet factor 4, HIT, immune, coagulopathy, thrombosis, novel coronavirus 2019, COVID-19, SARS-CoV-2, and 2019-nCoV.
The inclusion criteria for eligible studies were as follows: (1) individual case reports or case series including less than 20 adult patients (age ≥ 18 years) who were hospitalized for COVID-19 and confirmed HIT using the following platelet activation assays: serotonin release assay (SRA), heparin-induced platelet activation (HIPA) test, platelet aggregation test, or flow cytometric assay; or (2) randomized controlled trials, retrospective, or prospective observational studies enrolling at least 20 adult patients who were hospitalized for COVID-19 with reported incidence of HIT or sufficient data for computing the incidence of HIT. Nonoriginal articles (such as reviews, commentaries, or guidelines) and duplicated studies were excluded. Two authors (N.U. and N.T.) independently searched the literature, screened titles and abstracts, and reviewed full texts to identify potentially eligible studies. Disagreements were resolved by consensus or a third reviewer (T.C.) when necessary. The selection result was reported according to the PRISMA flowchart.
Data extraction
Two authors (N.U. and N.T.) independently reviewed full data from individual selected studies including supplementary materials and independently extracted prespecified data. Disagreements of extracted data were resolved by consensus or a third reviewer (T.C.) when necessary. The primary outcome was clinical and laboratory characteristics and clinical outcomes of patients with COVID-19 with confirmed HIT. The secondary outcomes were the incidence of HIT, the incidence of anti-PF4/H Ab detection, and risks associated with HIT development in hospitalized patients with COVID-19.
For each study, the following data were extracted: study design, study population, number of participants, baseline characteristics of patients (age, sex, and severity), heparin administration (indications, types, intensity, and duration of heparin exposure before HIT diagnosis), initial platelet counts, nadir platelet counts, thromboembolic events after heparin initiation, clinical scoring systems, screening immunoassays for HIT, confirmatory assays for HIT, alternative nonheparin anticoagulants, bleeding events, platelet transfusion, platelet recovery after nonheparin anticoagulants, patient’s outcomes, the incidence of confirmed HIT, and the frequency of anti-PF4/H Ab detection.
Quality assessment
The methodologic quality of included studies for meta-analysis was performed independently by 2 authors (N.U. and N.T.) using a validated tool for assessing studies reporting prevalence data.24 The tool contains 10 items assessing the external validity and internal validity of the study. For each item, a score of 0 or 1 was assigned to the answers yes or no, respectively. The summary assessment of overall risk of bias was rated according to the responses to the 10 items, and included studies were classified based on the total score as low (0-3), moderate (4-6), or high risk (7-10) of bias.
Data analysis
The meta-analysis was performed using Comprehensive Meta-analysis (Version 2; Biostat, Englewood, NJ). The pooled incidence of each outcome was calculated using DerSimonian and Laird method with random-effects model and were reported as the pooled incidence with 95% confidence interval (CI). Statistical heterogeneity was assessed using I2 statistic, which measured the inconsistency across study results. Interstudy heterogeneity was assigned as insignificant (I2 = 0% to 25%), low (I2 = 26% to 50%), moderate (I2 = 51% to 75%), and high (I2 > 75%).25 The funnel plot for evaluation of publication bias was not performed because of the low number of studies included in the meta-analysis (<10 studies). For descriptive statistics, normality of the data was tested using the Shapiro-Wilk test. Continuous data were presented as means (±standard deviations) or medians with interquartile ranges (IQRs) as appropriate. All descriptive analyses were computed using SPSS version 22.0 for Window (SPSS Inc., Chicago, IL).
Results
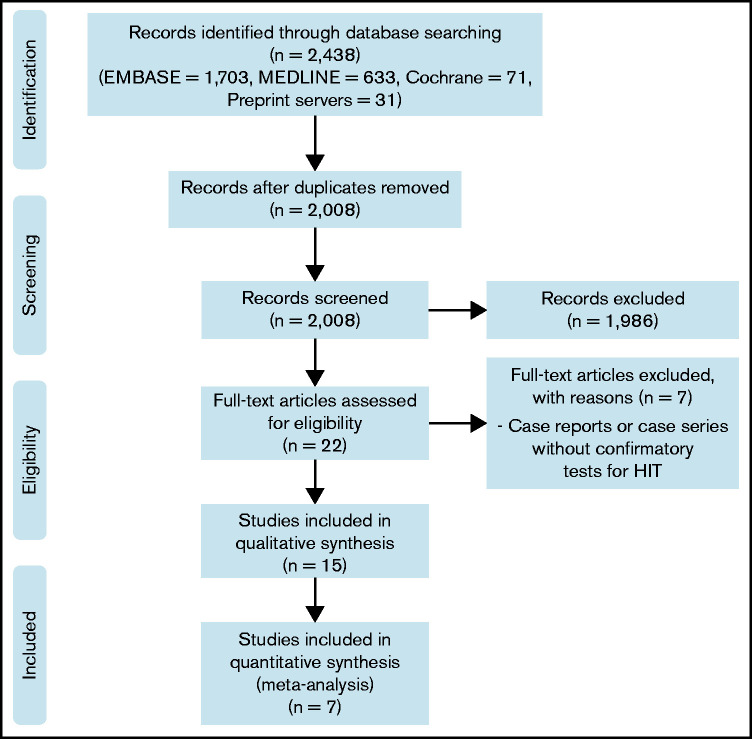
The PRISMA flow diagram is shown in Figure 1. A total of 2008 unique studies were identified by literature search and were screened by titles and abstracts. Of these, 1986 were excluded, and 22 full texts were screened for eligibility. Eventually, 15 studies26-40 met the eligibility criteria and were included in qualitative synthesis, and 7 studies29,32-36,40 were sufficient for quantitative synthesis. Of 7 studies eligible for meta-analysis, the risks of bias were individually assessed. Four studies33-35,40 were assigned as low risk of bias, whereas the other 3 studies29,32,36 were classified as moderate risk of bias (supplemental Table 1).
Figure 1.
PRISMA flow diagram.
Study characteristics
The main characteristics of the 15 included studies (12 published full-texts, 1 full-preprint report, and 2 abstracts)26-40 are summarized in Table 1. Across 15 studies (8053 total patients), there were a total of 40 reported HIT patients. Of these, 19 HIT cases and 1 with autoimmune HIT who had diagnosis confirmed by SRA or HIPA were included for clinical and laboratory characterization.26-33,37-39 From 6 concurrent cohorts, clinical and laboratory data of 26 patients who were suspected to have HIT but had negative confirmatory tests were available for comparison.26-29,33,37
Table 1.
Characteristics of included studies
| Reference (first author and year) | Study design | N | Study population | Pretest clinical scoring system | Immunoassay for screening HIT | Platelet activation assay for confirming HIT | Suspected HIT/confirmed HIT | Proportion of ICU or critical illness | Heparin administration in confirmed HIT |
|---|---|---|---|---|---|---|---|---|---|
| Riker26 2020 | Case report | 16 | Thrombocytopenia with anti-PF4 Ab among intubated COVID-19 patients with ARDS | 4T score | ELISA | SRA | 3/1 | 16 (100%) | 2 prophylactic UFH/LMWH and 1 therapeutic UFH |
| Lingamaneni27 2020 | Case report | 5 | COVID-19 patients with HIT suspicion | 4T score | ELISA | SRA | 5/1 | 5 (100%) | All 5 received therapeutic heparin |
| May28 2020 | Case report | 7 | Hospitalized COVID-19 patients with positive anti-platelet factor 4 Ab | 4T score | ELISA | SRA | 7/1 | 7 (100%) | 3 prophylactic UFH, 3 prophylactic LMWH and 1 prophylactic LMWH/UFH |
| Patell29 2020 | Retrospective cohort | 88 | patients hospitalized with Covid-19 and received intravenous UFH for ≥5 d |
4T score | Latex immune turbidimetric assay | SRA | 8/3 | NR | All 3 confirmed HIT received therapeutic UFH |
| Bidar30 2020 | Case report | 2 | Confirmed HIT in COVID-19 patients with severe ARDS on VVECMO | NR | ELISA | HIPA | 2/2 | 2 (100%) | All 2 confirmed HIT received therapeutic UFH |
| Tran31 2020 | Case report | 1 | A patient with SARS-CoV-2 pneumonitis and confirmed HIT | 4T score | ELISA | HIPA | 1/1 | 1 | The patient with confirmed HIT received prophylactic LMWH |
| Daviet32 2020 | Retrospective cohort | 86 | COVID-19 ARDS in 2 ICUs enrolled in COAG-COVID trial | 4T score | Quantitative CIA; IgG specific | HIPA | NR/7 | 86 (100%) | All 7 confirmed HIT received therapeutic LMWH or UFH |
| Delrue33 2020 | Retrospective cohort | 626 | All consecutive SARS-CoV-2-infected adults admitted to the ICU and medical wards | 4T score | PaGIA, ELISA IgG | HIPLA, SRA | 10/1 | 184 (29.4%) | Of 10 HIT suspicions, 2 received UFH, 1 received LMWH and 7 received LMWH followed by UFH |
| Helms34 2020 | Prospective cohort | 150 | All patients with SARS-CoV-2 ARDS admitted to the ICU | NR | NR | NR | 4/0 | 150 (100%) | 150 (100%); 105 (70%) prophylactic doses; 45 (30%) therapeutic doses |
| Ionescu35 2020 | Retrospective cohort | 3480 (2574 receiving LMWH or UFH) | Consecutive COVID-19 adult patients hospitalized within 8 hospitals located in Southeast Michigan | NR | NR | NR | NR/12 | 642 (18.4%) | 1156 with prophylactic LMWH; 699 with received prophylactic UFH; 424 with therapeutic LMWH and 295 with therapeutic UFH |
| Santi36 2020 | Retrospective cohort | 94 | Hospitalized patients infected with COVID-19 |
NR | NR | NR | NR/2 | NR | 2 received therapeutic UFH |
| Warrior37 2020 | Retrospective cohort | 1265 | Hospitalized COVID-19 positive patients | 4T score | ELISA | SRA | 8/1 | NR | Of 8 HIT suspicions, 4 received LMWH, 2 received UFH and 2 received LMWH followed by UFH |
| Madala38 2021 | Case report | 1 | A patient with SARS-CoV-2 pneumonia and ischemic stroke | 4T score | ELISA | SRA | 1/1 | 1 | The patient with confirmed HIT received UFH followed by LMWH |
| Julian39 2021 | Case report | 1 | A patient with COVID-19 positive and confirmed autoimmune HIT | N/A | ELISA | SRA | 1/1 | 1 | No heparin exposure before documented thrombosis and thrombocytopenia |
| Lawler40 2021 | Randomized controlled trial | 2231 | Non-critically ill patients hospitalized for Covid-19 |
4T score | ELISA | SRA | NA/0 | 0 | 1181 received therapeutic- and 1050 received prophylactic-dose anticoagulation; no confirmed HIT |
Ab, antibody; CIA, chemiluminescent immunoassay; ELISA (or EIA), enzyme-linked immunosorbent assay (or enzyme immunoassay); ICU, intensive care unit; NR, not reported; PaGIA, particle gel immunoassay.
A total of 7 studies were included for the estimation of the pooled incidence of HIT.29,32-36,40 These 7 studies collectively included a total of 5849 patients, ranging from 86 to 2574 patients.
Clinical and laboratory characteristics of confirmed HIT in hospitalized patients with COVID-19
From 11 studies, there were 19 confirmed HIT cases and 1 with autoimmune HIT, with sufficient data for characterization.26-33,37-39 Clinical and laboratory characteristics of individual cases are summarized in Table 2. Among the 19 documented cases of HIT, the median age was 62.0 (IQR, 51.0, 64.0) years. Males were the predominant proportion (74%). All but 1 patient (95%) were critically ill and were admitted to the ICU. Nine patients received UFH, 2 received LMWH, and 8 received both LMWH and UFH. Of the 18 patients with available data on heparin intensity, 13 (72%) received therapeutic-intensity anticoagulation, whereas 5 (28%) received prophylactic anticoagulation. The median time from heparin initiation to HIT diagnosis was 13.5 (IQR, 10.75, 16.25) days. Median baseline platelet counts and median nadir platelet counts were 223 × 109/L (IQR, 160.5, 297) and 56 × 109/L (IQR, 37, 73), respectively. All but 1 patient had intermediate or high pretest probability for HIT using the 4T scoring system, with a median of 5.5 (lQR, 4, 6). Twelve patients (63%) developed thrombosis after heparin administration. A total of 15 of 19 (79%) confirmed HIT cases demonstrated platelet recovery after heparin substitution with nonheparin anticoagulants. Of the 17 with known survival outcomes, 4 died shortly after HIT diagnosis.
Table 2.
Clinical and laboratory characteristics of hospitalized patients with COVID-19 with confirmed HIT
| Case | Age (y), sex | Severity of COVID-19 | Indication of heparin | Type and dose of heparin | Duration of heparin to HIT diagnosis (d) | Initial platelet count (x109 /L) | Nadir platelet count (x109 /L) | Thrombosis after heparin initiation | 4T score | Screening test | Confirmatory test | Non-heparin anticoagulants | Platelet response after HIT treatment | Outcomes of patients as reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 126 | 70, M | ICU (MV, ARDS) | DVT prophylaxis | UFH; prophylaxis | 20 | 438 | 90 | PE | 6 | ELISA (OD 2.0) | SRA, positive (48%) | Bivalirudin | Death shortly after HIT diagnosis | Death |
| 227 | 63, M | ICU (MV) | DVT prophylaxis | LMWH; prophylaxis | 12 | 304 | 96 | DVT | 6 | ELISA (OD 1.2) | SRA, positive (49%) | Argatroban | Death shortly after HIT diagnosis | Death |
| 328 | 61. F | ICU (RRT) | RRT | UFH; prophylactic | N/A | N/A | 37 | N/A | 4 | ELISA (OD 0.95) | SRA, positive | N/A | N/A | N/A |
| 429 | 68, F | ICU | AF | UFH; therapeutic | 7 | 416 | <5 | None | 4 | LITA (1.8 U/mL) | SRA, positive | Argatroban, bivalirudin | Platelet recovery | Alive |
| 529 | 63, M | ICU | STEMI | UFH; therapeutic | 6 | 154 | 51 | Splenic infarct and cerebral infarct | 8 | LITA (1.6 U/mL) | SRA, borderline positive | Argatroban | Platelet recovery | Death |
| 629 | 49, M | ICU | COVID pneumonia | UFH; therapeutic | 12 | 176 | 25 | None | 6 | LITA (1.9 U/mL) | SRA, borderline positive | Argatroban | Platelet recovery | Alive |
| 730 | 62. F | ICU (MV, ECMO) | PE, ECMO | UFH; therapeutic | 16 | 237 | 29 | None | 3 | ELISA (OD 1.8) | HIPA, positive | Argatroban | Platelet recovery, discharge from hospital | Alive |
| 830 | 38, M | ICU (MV, ECMO) | ECMO | UFH; therapeutic | 21 | 248 | 50 | None | 4 | ELISA (OD 1.6) | HIPA, positive | Argatroban | Platelet recovery, discharge from hospital | Alive |
| 931 | 62, M | ICU (MV) | VTE prophylaxis | LMWH and UFH flush; prophylactic | 17 | 412 | 91 | PE | 4 | ELISA (OD 1.1) | HIPA, positive | Bivalirudin | Platelet recovery | Alive |
| 1032 | 46, M | ICU (ARDS. MV, ECMO) | Clinical trial (COAG-COVID)a | LMWH and UFH; therapeutic | 16 | 61 | 33 | Multiple DVT | 6 | CIA (46 U/mL) | HIPA, positive | Argatroban | Platelet recovery/ discharge from ICU | Discharge from ICU |
| 1132 | 50, M | ICU (ARDS, MV, ECMO) | Clinical trial (COAG-COVID)a | LMWH and UFH; therapeutic | 13 | 243 | 73 | Intracardiac thrombus, ECMO membrane thrombosis | 6 | CIA (11 U/mL) | HIPA, positive | Argatroban | Platelet recovery/ still in ICU | Still in ICU |
| 1232 | 43, F | ICU (ARDS, MV, ECMO) | Clinical trial (COAG-COVID)a | LMWH and UFH; therapeutic | 15 | 160 | 48 | Multiple DVT, ECMO pump thrombosis | 6 | CIA (39 U/ML) | HIPA, positive | Argatroban | Platelet recovery/ still in ICU | Still in ICU |
| 1332 | 63, M | ICU (ARDS, MV) | Clinical trial (COAG-COVID)a | LMWH and UFH; therapeutic | 14 | 191 | 56 | Stroke | 4 | CIA (60 U/mL) | HIPA, positive | Danaparoid | Platelet recovery, discharge from hospital | Alive |
| 1432 | 59, M | ICU (ARDS, MV) | Clinical trial (COAG-COVID)a | LMWH and UFH; therapeutic | 9 | 161 | 62 | DVT | 5 | CIA (4 U/mL) | HIPA, positive | Danaparoid | Platelet recovery, discharge from ICU | Discharge from ICU |
| 1532 | 57, M | ICU (ARDS) | Clinical trial (COAG-COVID)a | UFH; therapeutic | 11 | 159 | 39 | None | 5 | CIA (21 U/mL) | HIPA, positive | Danaparoid | Platelet recovery, discharge from hospital | Alive |
| 1632 | 69, M | ICU (ARDS, MV) | Clinical trial (COAG-COVID)a | UFH; therapeutic | 16 | 215 | 107 | None | 4 | CIA (2 U/mL) | HIPA, positive | Danaparoid | Platelet recovery, discharge from hospital | Alive |
| 1733 | 64, M | ICU | VTE prophylaxis | LMWH; prophylactic | 18 | 223 | 67 | DVT | 6 | PaGIA positive, ELISA IgG (OD 2.4) | HIPLA, positive (75%), SRA, positive (94% at 0.1 U/mL and 103% at 0.5 U/mL heparin) | Argatroban (13 d), danaparoid (19 d), apixaban at discharge | Platelet recovery, discharge from hospital | Alive |
| 1837 | 63, M | ICU (RRT) | RRT | LMWH and UFH; NA | N/A | N/A | 67 | PE | ≥4 | ELISA IgG (OD 0.62) | SRA, positive | Argatroban | N/A | Death |
| 1938 | 65, F | Non-ICU | AF | LMWH and UFH; therapeutic | 12 | 290 | 63 | Stroke, PE, iliac and femoral artery thrombosis, | 6 | CIA (9.7 U/mL) | SRA, positive (94%) | Argatroban, apixaban | Platelet recovery, discharge from hospital | Alive |
| 2039 | 65, M | Non-ICU | N/A | N/A | N/A (8 d after diagnosis of COVID-19) |
N/A | 6 | DVT, PE (at presentation) | N/A | NR, positive | SRA, positive | Argatroban, apixaban, IVIG | Platele recovery, discharge from hospital | Alive |
ARDS, acute respiratory distress syndrome; CIA, chemiluminescent immunoassay (cutoff < 1 U/mL); DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; ELISA, enzyme-link immunosorbent assay; F, female; HIPLA, heparin-induced platelet activation assay (positivity threshold 13%); ICU, intensive care unit; LITA, latex immune turbidimetric assay; M, male; MV, mechanical ventilation; N/A, not available; PE, pulmonary embolism; RRT, renal replacement therapy; TE, thromboembolism; VTE, venous thromboembolism.
COAG-COVID (Coagulopathy of COVID-19: A Pragmatic Randomized Controlled Trial of Therapeutic Anticoagulation Versus Standard Care).
Only 1 patient with COVID-19 with autoimmune HIT was identified.39 He presented with pulmonary embolisms coexisting with severe thrombocytopenia 8 days after diagnosis of COVID-19 without previous heparin exposure, leading to a high suspicion of autoimmune HIT, which was confirmed by presence of functional anti-PF4/H Abs using SRA. He was successfully treated with intravenous immunoglobulin and argatroban, followed by apixaban.
In 6 concurrent studies, hospitalized patients with COVID-19 who were suspected to have HIT but had negative confirmatory tests were reported.26-29,33,37 Among these, 26 cases were reviewed for comparison with confirmed HIT (Table 3). Clinical and laboratory variables of patients with confirmed HIT and patients with negative HIT confirmatory tests are summarized (Table 4). The variables of both groups were closely similar. Sixteen patients (61.5%) developed thromboembolic events after heparin therapy. Of patients with known survival outcomes, 13 of 20 (65%) patients died shortly after suspected HIT.
Table 3.
Clinical and laboratory characteristics of hospitalized patients with COVID-19 with negative confirmatory tests for heparin-induced thrombocytopenia
| Case | Age (y), sex | Severity of COVID-19 | Indication of heparin | Type and dose of heparin during HIT diagnosis | Duration of heparin when tested for HIT (d) | Initial platelet count (x109 /L) | Nadir platelet count (x109 /L) | Thrombosis after heparin initiation | 4T score | Screening test | Confirmatory test | Non-heparin anticoagulants | Platelet recovery after HIT treatment | Patients’ outcomes as reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 126 | 74, M | ICU (MV, ARDS) | DVT prophylaxis | LMWH and UFH; prophylactic | 12 | 143 | 68 | Upper extremity venous thrombosis | 4 | ELISA (OD 1.3) | SRA, negative (0%) | Fondaparinux then bivalirudin | None | Death |
| 226 | 53, M | ICU (MV, ARDS) | AF | UFH; therapeutic | 11 | 207 | 22 | Skin necrosis | 6 | ELISA (OD 0.48) | SRA negative (0%) | Argatroban then apixaban | Recovery | Alive |
| 327 | 53, M | ICU (ARDS) | ACS and AF | N/A | 7 | N/A | N/A | None | 5 | ELISA (OD 0.71) | SRA negative | Argatroban | N/A | N/A |
| 427 | 61. F | ICU (ARDS) | DVT | N/A | 6 | N/A | N/A | DVT | 7 | ELISA (OD 0.77) | SRA, negative | N/A | N/A | N/A |
| 527 | 68, F | ICU (ARDS) | DVT | N/A | 8 | N/A | N/A | DVT | 7 | ELISA (OD 0.42) |
SRA, negative | N/A | N/A | N/A |
| 627 | 63, M | ICU (ARDS) | Suspected PE | N/A | 2 | N/A | N/A | Suspected PE | 4 | ELISA (OD 0.31) | SRA, negative | N/A | N/A | N/A |
| 728 | 50, M | ICU (ECMO) | ECMO | UFH; prophylactic | N/A | N/A | 49 | None | 5 | ELISA (OD 0.63) | SRA, negative | N/A | N/A | Death |
| 828 | 79, F | N/A | VTE prophylaxis | LMWH; prophylactic | N/A | N/A | 155 | None | 3 | ELISA (OD 1.89) | SRA, negative | N/A | N/A | Alive |
| 928 | 58, F | N/A | VTE prophylaxis | LMWH; prophylactic | N/A | N/A | 305 | PE | 3 | ELISA (OD 0.51) | SRA, negative | N/A | N/A | Death |
| 1028 | 38, M | ICU (ECMO) | VTE prophylaxis, ECMO | LMWH and UFH; prophylactic | N/A | N/A | 39 | None | 3 | ELISA (OD 0.83) | SRA, negative | N/A | N/A | N/A |
| 1128 | 71, F | ICU (RRT) | RRT | UFH; prophylactic | N/A | N/A | 70 | Stroke | 6 | ELISA (OD 0.47) | SRA, negative | N/A | N/A | Death |
| 1228 | 46, M | N/A | VTE prophylaxis | LMWH; prophylactic | N/A | N/A | 59 | DVT | 5 | ELISA (OD 0.83) | SRA, negative | N/A | N/A | N/A |
| 1329 | 49, M | ICU | COVID pneumonia | UFH; therapeutic | 6 | 211 | 47 | None | 6 | LITA (1.1 U/mL) | SRA, negative | Argatroban | None | Death |
| 1433 | 77, M | ICU | VTE prophylaxis | LMWH and UFH; prophylactic | 11 | 136 | 59 | None | 5 | PaGIA, negative | HIPLA, negative | None | N/A | Death |
| 1533 | 63, M | ICU | VTE prophylaxis | LMWH and UFH; therapeutic | 14 | 250 | 11 | None | 4 | PaGIA, negative | HIPLA, negative | None | N/A | Alive |
| 1633 | 60, M | ICU | VTE prophylaxis and DVT treatment | LMWH and UFH; therapeutic | 21 | 153 | 36 | DVT | 4 | PaGIA, negative | HIPLA, negative | None | N/A | Death |
| 1733 | 63, M | ICU | AF | LMWH and UFH; therapeutic | 12 | 177 | 38 | None | 4 | PaGIA, negative | HIPLA, negative | None | N/A | Death |
| 1833 | 71, M | ICU | AF | LMWH and UFH; therapeutic | 21 | 240 | 77 | None | 4 | PaGIA, negative | HIPLA, negative | None | N/A | Death |
| 1933 | 66, M | ICU | PE | UFH; therapeutic | 2 | 121 | 59 | PE and DVT | 4 | PaGIA, negative | HIPLA, negative | None | N/A | Alive |
| 2033 | 50, M | ICU | VTE prophylaxis | LMWH; prophylactic | 12 | 227 | 136 | Stroke | 6 | PaGIA negative |
HIPLA, negative | None | N/A | Alive |
| 2133 | 67, M | ICU | AF | UFH; therapeutic | 23 | 363 | 138 | DVT | 6 | PaGIA, negative | HIPLA, negative | None | N/A | Death |
| 2233 | 65, M | ICU | PE suspicion | LMWH and UFH; therapeutic | 24 | 317 | 138 | PE and DVT | 6 | PaGIA, negative | HIPLA, negative | Argatroban | N/A | Death |
| 2337 | 58, M | N/A | N/A | LMWH; N/A | N/A | N/A | 60 | DVT | ≥4 | ELISA IgG (OD 1.68) | SRA, negative | Argatroban | N/A | Death |
| 2437 | 77, M | ICU (RRT) | N/A | LMWH and UFH; N/A | N/A | N/A | 28 | Stroke | ≥4 | ELISA IgG (0.7) | SRA, negative | Argatroban | N/A | Alive |
| 2537 | 36, M | N/A | N/A | LMWH; N/A | N/A | N/A | 2 | None | ≥4 | ELISA IgG (0.88) | SRA, negative | Argatroban | N/A | Alive |
| 2637 | 34, M | N/A | N/A | LMWH; N/A | N/A | N/A | 65 | DVT | ≥4 | N/A | SRA, negative | Bivalirudin | N/A | Death |
ARDS, acute respiratory distress syndrome; CIA, chemiluminescent immunoassay (cutoff < 1 U/mL); DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; ELISA, enzyme-link immunosorbent assay; F, female; HIPLA, heparin-induced platelet activation assay (positivity threshold 13%); ICU, intensive care unit; LITA, latex immune turbidimetric assay; M, male; MV, mechanical ventilation; N/A, not available; PE, pulmonary embolism; RRT, renal replacement therapy; TE, thromboembolism; VTE, venous thromboembolism.
Table 4.
Clinical characteristics and laboratory profiles of hospitalized patients with COVID-19 with confirmed HIT and patients with negative confirmatory tests
| Variables | Patients with confirmed HIT (N = 19) | Patients with negative confirmatory tests (N = 26) |
|---|---|---|
| Age (y) | 62.0 (50.0, 64.0)* | 62 (50, 68.75)* |
| Sex (male; female) | 14 (74%); 5 (26%) | 21 (81%); 5 (19%) |
| Type of heparins (UFH; LMWH) | 17 (63%); 10 (37%) | 15 (43%), 16 (46%) [4 N/A (11%)] |
| Intensity of anticoagulants (prophylactic; therapeutic) | 5 (26.3%); 13 (68.4%) [1 N/A (5.3%)] | 9 (34.6%%); 9 (34.6%); [8 N/A (30.8%)] |
| Duration of heparin to HIT diagnosis (d) | 13.5 (10.75, 16.25)* | 11 (6, 21)* |
| Initial platelet counts (×109/L) | 223 (160.5, 297)* | 209 (145.5, 247.5)* |
| Nadir platelet counts (×109/L) | 56 (37, 73)* | 59 (37.5, 91.75)* |
| Thrombosis after heparin administration | 12 (63%) | 16 (61.5%) |
| 4T score | 5.5 (4, 6)* | 5 (4, 6)* |
N/A, not available.
Median (interquartile range).
Incidence of heparin-induced thrombocytopenia in hospitalized patients with COVID-19
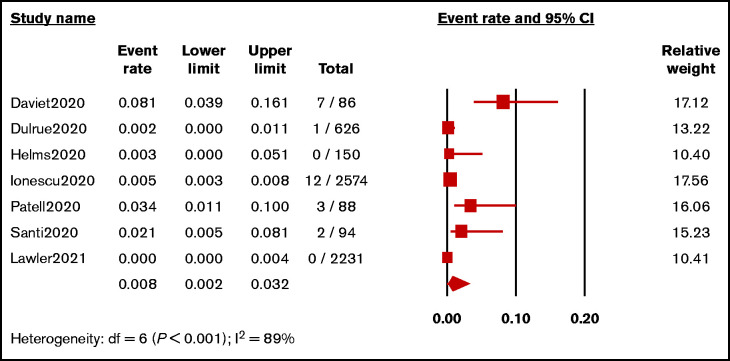
From 7 studies (N = 5849 patients),29,32-36,40 the pooled incidence of HIT in hospitalized patients with COVID-19 was 0.8% (95% CI, 0.2%-3.2%; I2 = 89%; Figure 2). A sensitivity analysis of 4 studies (N = 3031),29,32,33,40 in which the diagnostic criteria for HIT were specified, revealed the pooled incidence of 0.8% (95% CI, 0.1%-6.4%; I2 = 89%; supplemental Figure 1).
Figure 2.
Forest plot showing pooled estimated incidence of HIT in hospitalized patients with COVID-19.
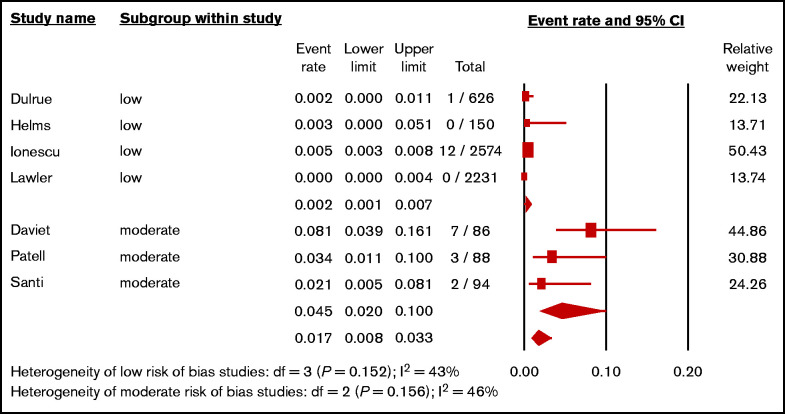
A subgroup analysis according to the study’s risk of bias was performed (Figure 3). The pooled incidence of 3 small studies29,32,36 with moderate risk of bias, which included 268 patients mostly receiving UFH, was 4.5% (95% CI, 2.0%-10.0%; I2 = 46%). The pooled incidence of 4 larger studies33-35,40 with low risk of bias (5581 patients) was 0.2% (95% CI, 0.1%-0.7%; I2 = 43%). There was a significant difference between the low risk and moderate risk of bias (P < .001).
Figure 3.
Forest plot showing the pooled estimated incidence of HIT in hospitalized patients with COVID-19 according to the risks of bias.
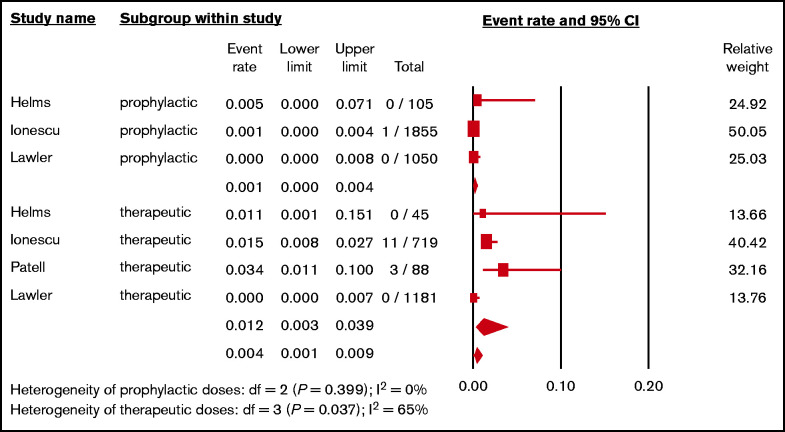
Data on the incidence of HIT stratified by anticoagulation intensity were available in 4 studies.29,34,35,40 The pooled incidence of HIT in patients receiving prophylactic-intensity heparins was 0.1% (3 studies; N = 3010; 95% CI, 0.0%-0.4%; I2 = 0%),34,35,40 whereas the pooled incidence of HIT in patients receiving therapeutic heparins was 1.2% (4 studies; N = 2033; 95% CI, 0.3%, 3.9%; I2 = 65%)29,34,35,40 (Figure 4). The pairwise comparison revealed significant difference between prophylactic vs therapeutic heparins (P = .007). We also analyzed the difference of HIT in the largest cohort including 2574 patients receiving heparins.35 HIT development was higher in patients receiving therapeutic anticoagulation compared with prophylactic anticoagulation (odds ratio, 28.8; 95% CI, 3.7-223.5; P = .001).
Figure 4.
Forest plot showing the pooled estimated incidence of HIT in hospitalized patients with COVID-19 according to the intensities of heparins.
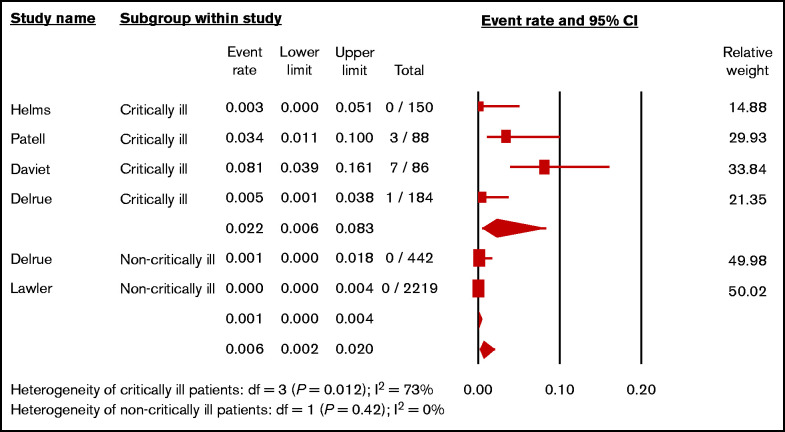
A subgroup analysis to estimate pooled incidences of HIT in critically ill patients with COVID-19 and non–critically ill patients with COVID-19 was performed. A total of 3169 patients from 5 studies29,32-34,40 were available for analysis. The pooled incidence of HIT in critically ill patients with COVID-19 was 2.2% (4 studies; N = 508; 95% CI, 0.6%-8.3%; I2 = 73%),29,32-34 whereas the pooled incidence of HIT in non–critically ill patients with COVID-19 was 0.1% (2 studies; N = 2661; 95% CI, 0.0%-0.4%; I2 = 0%)33,40 (Figure 5). The pairwise comparison revealed significant difference between critically ill and non–critically ill patients with COVID-19 (P = .002).
Figure 5.
Forest plot showing the pooled estimated incidence of HIT in hospitalized patients with COVID-19 according to severity of patients.
Although the prespecified subgroup analysis to assess the risk for HIT between UFH and LMWH was planned, there were not sufficient data to compute the pooled incidences of HIT for UFH and LMWH.
Incidence of anti-PF4/H Abs detection in hospitalized patients with COVID-19
Existing data were not sufficient for estimating the pooled incidence of anti-PF4/H Abs (activating and nonactivating) in all hospitalized patients with COVID-19 receiving heparins, because the tests were only performed based on suspicion of HIT. In the only 1 study whereby anti-PF4/H Abs were screened in 172 consecutive patients (64 ICU and 108 non-ICU),33 the frequency of anti-PF4/H-associated polyspecific Abs (immunoglobulin M [IgM], IgA, and IgG; OD of >0.5) was 33%, whereas the frequency of anti-PF4/H–associated monospecific IgG (OD of >0.5) was 16%. Of the 19 cases with anti-PF4/H–associated polyspecific Abs with an OD of >1.0, 7 (37%) patients had thromboembolic events. However, all patients with positive anti-PF4/H Abs yielded negative HIT confirmatory tests.
Discussion
In this systematic review, we characterized 19 hospitalized patients with COVID-19 with confirmed HIT using standard platelet activation assays.26-33,37,38 There were 26 patients who had suspected HIT but had negative confirmatory tests from concurrent cohorts for comparison. Some cases were assigned as HIT in their original cohorts because of strongly positive immunoassays.26,29,37 However, a recent study revealed that patients with COVID-19 frequently had strongly positive immunoassays without platelet-activating antibodies indicating nonpathogenic antibodies.41 Therefore, HIT diagnosis requires confirmatory heparin-dependent platelet-activating tests despite the strongly positive immunoassays to avoid overdiagnosis and overtreatment of HIT.
Of the 19 confirmed cases of HIT, most patients were critically ill in the ICU, and males were predominant. The overrepresentation of males in patients with COVID-19 with suspected HIT may be explained by the higher proportion of male patients admitted in the ICU and the higher probability to develop thrombocytopenia triggering investigation for HIT.42
Half of patients were diagnosed after 14 days of heparin exposure, suggesting a delay in diagnoses of HIT in patients with COVID-19. Most of the patients with suspected HIT obtained a 4T score of ≥4. In addition, a similar proportion of confirmed HIT (12 of 19, 63%) and suspected HIT (16 of 26, 61.5%) cases developed thrombosis after heparin administration. Therefore, it is apparent that clinical features such as platelet counts and the presence of thrombosis cannot reliably predict the diagnosis of HIT in patients with COVID-19, and functional tests are required for definitive diagnosis.
Almost all patients with confirmed HIT had platelet recovery shortly after switching to nonheparin anticoagulants. When the causes of thrombocytopenia are not obvious, immediate platelet response to alternative anticoagulant may be suggestive of HIT diagnosis. This observation may be helpful while waiting for a confirmatory assay, which may not be readily available for timely clinical management.
In this meta-analysis, we report the pooled incidence of HIT in hospitalized patients with COVID-19 of 0.8% (95% CI, 0.2%-3.2%), which was comparable to those reported from large cohorts and meta-analysis of medical patients without COVID-19; the incidences of HIT ranged from 0.08% to 0.94%.43 However, there was high heterogeneity among studies because 3 small cohorts with moderate risk of bias29,32,36 had very high incidence of HIT in patients with COVID-19 (4.5%; 95% CI, 2%-10%). All confirmed HIT in these 3 cohorts received therapeutic doses of UFH. In contrast, the pooled incidence of HIT from 4 larger cohorts33-35,40 with low risks of bias was 0.2% (95% CI, 0.1%-0.7%) similar to patients without COVID-19. Therefore, prospective systematic studies are warranted to determine the incidence of HIT among different COVID-19 populations (based on disease severity, types of heparin, and heparin dosing).
In critically ill patients, nonactivating anti-PF4/H Abs may be present without causing HIT. In these cases, the screening immunologic assays are positive with negative confirmatory tests. In our review, there was only 1 study that screened anti-PF4/H Abs in consecutive hospitalized patients with COVID-19.33 Compared with the HIT incidence of 0.2% (95% CI, 0%-1.1%), the frequency of patients with detected anti-PF4/H Abs was substantially higher (33%).33 Similarly to cases without COVID-19, HIT developed in less than 10% of patients with detectable anti-PF4/H Abs.44 Therefore, the routine screening for anti-PF4/H Abs in patients with COVID-19 receiving heparin is probably not cost efficient.
The prespecified subgroup analysis was performed to assess the risk of heparin intensity and HIT development in COVID-19. Hospitalized patients with COVID-19 who received therapeutic doses of heparins were at greater risk for HIT than those who received prophylactic doses of heparins.29,34,35,40 The recent INSPIRATION randomized controlled trial failed to demonstrate the benefits of intermediate-dose anticoagulation compared with prophylactic-dose anticoagulation to reduce thrombosis or mortality in patients in the ICU with COVID-19.19 Notably, 7 thrombocytopenias of unspecified causes occurred only in patients assigned to the intermediate-dose group, with an absolute risk difference of 2.2% (95% CI, 0.4%-3.8%; P = .01). Therefore, the risk of HIT in higher-intensity anticoagulation should be considered.
The estimated incidence of HIT in critically ill patients with COVID-19 was 2.2%. This was relatively higher than those previously reported in patients without COVID-19 (0.3%-0.5%).21,45 The hyperactivation of the immune system in COVID-19 may activate platelets to release of PF4 into circulation and stimulate anti-PF4/H Ab production.46 Severe endothelial injury, platelet hyperactivation, and immune dysregulation after SARS-CoV-2 infection may involve in development of HIT in critically ill patients with COVID-19.47
There are some limitations of this study. Most of studies were case reports, small case series, or retrospective cohorts, which are prone to biases because of different HIT confirmatory tests and criteria, lack of central adjudication of HIT cases, and incomplete data collection. In addition, all but 1 cohort did not perform systematic surveillance, which may lead to either under- or overestimation of the incidences of HIT in patients with COVID-19 because of case selection, as well as significant heterogeneity among studies. Finally, the number of studies included in both qualitative and quantitative analyses was relatively small.
Conclusions
In this systematic review and meta-analysis, we reported a pooled incidence of HIT in patients with COVID-19 of 0.8%, which was similar to those previously reported in medical patients without COVID-19. However, the incidence of HIT in patients with COVID-19 might be increased in patients receiving therapeutic-dose heparin and in critically ill patients. The clinical and laboratory profiles between patient with COVID-19 with confirmed HIT and suspected HIT with negative confirmation were similar. A large prospective cohort with systematic surveillance of HIT is required to estimate the true incidence and determine risk factors for HIT in hospitalized patients with COVID-19.
Supplementary Material
The full-text version of this article contains a data supplement.
Authorship
Contribution: N.U. was involved in conceptualization, database search, screening of abstracts and full texts, data extraction and analysis, quality appraisal, and writing the original draft and revision of manuscript; N.T. was involved in conceptualization, database search, screening of abstracts and full texts, and editing of the manuscript and revised manuscript; P.R. was involved in data analysis, appraisal, and editing of the manuscript and revised manuscript; R.P. was involved in data analysis and editing of the manuscript and revised manuscript; J.I.Z. was involved in appraisal and editing of the manuscript and revised manuscript; and T.C. was involved in conceptualization, adjudication, data analysis, and editing of the manuscript and revised manuscript.
Conflict-of-interest disclosure: J.I.Z. reports research funding from Incyte and Quercegen; consultancy services to Sanofi, CSL, and Parexel; and honoraria from/advisory board participation with Pfizer/Bristol Myers Squibb, Portola, Daiichi, Sanofi, and CSL Behring. All remaining authors declare no competing financial interests.
Correspondence: Noppacharn Uaprasert, Division of Hematology, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Rama IV Rd, Pathumwan, Bangkok 10330, Thailand; e-mail: drnoppacharn@yahoo.com.
References
- 1.Worldometer. COVID-19 coronavirus pandemic. Available at: https://www.worldometers.info/coronavirus/. Accessed 18 June 2021.
- 2.Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin S, Jin Y, Xu B, Hong J, Yang X.. Prevalence and impact of coagulation dysfunction in COVID-19 in China: a meta-analysis. Thromb Haemost. 2020;120(11):1524-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uaprasert N, Moonla C, Sosothikul D, Rojnuckarin P, Chiasakul T.. Systemic coagulopathy in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Clin Appl Thromb Hemost. 2021;27:1076029620987629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z.. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1994-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longchamp A, Longchamp J, Manzocchi-Besson S, et al. Venous thromboembolism in critically Ill patients with COVID-19: results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost. 2020;4(5):842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spyropoulos AC, Levy JH, Ageno W, et al. ; Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis . Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019. Chest. 2020;158(3):1143-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patell R, Chiasakul T, Bauer E, Zwicker JI.. Pharmacologic thromboprophylaxis and thrombosis in hospitalized patients with COVID-19: a pooled analysis. Thromb Haemost. 2021;121(1):76-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sholzberg M, Tang GH, Negri E, et al. Coagulopathy of hospitalised COVID-19: a pragmatic randomised controlled trial of therapeutic anticoagulation versus standard care as a rapid response to the COVID-19 pandemic (RAPID COVID COAG-RAPID Trial): a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group .Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadeghipour P, Talasaz AH, Rashidi F, et al. ; INSPIRATION Investigators . Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(3):252-261. [DOI] [PubMed] [Google Scholar]
- 21.East JM, Cserti-Gazdewich CM, Granton JT.. Heparin-induced thrombocytopenia in the critically ill patient. Chest. 2018;154(3):678-690. [DOI] [PubMed] [Google Scholar]
- 22.Uaprasert N, Chanswangphuwana C, Akkawat B, Rojnuckarin P.. Comparison of diagnostic performance of the heparin-induced thrombocytopenia expert probability and the 4Ts score in screening for heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2013;24(3): 261-268. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group .Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934-939. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riker RR, May TL, Fraser GL, et al. Heparin-induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. Res Pract Thromb Haemost. 2020;4(5):936-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingamaneni P, Gonakoti S, Moturi K, Vohra I, Zia M.. Heparin-induced thrombocytopenia in COVID-19. J Investig Med High Impact Case Rep. 2020;8:2324709620944091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May JE, Siniard RC, Marques M.. The challenges of diagnosing heparin-induced thrombocytopenia in patients with COVID-19. Res Pract Thromb Haemost. 2020;4(6):1066-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patell R, Khan AM, Bogue T, et al. Heparin induced thrombocytopenia antibodies in Covid-19 [published online ahead of print 13 July]. Am J Hematol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bidar F, Hékimian G, Martin-Toutain I, Lebreton G, Combes A, Frère C.. Heparin-induced thrombocytopenia in COVID-19 patients with severe acute respiratory distress syndrome requiring extracorporeal membrane oxygenation: two case reports. J Artif Organs. 2020;24(2):277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran M, Sheth C, Bhandari R, Cameron SJ, Hornacek D.. SARS-CoV-2 and pulmonary embolism: who stole the platelets? Thromb J. 2020;18(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daviet F, Guervilly C, Baldesi O, et al. Heparin-induced thrombocytopenia in severe COVID-19. Circulation. 2020;142(19):1875-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delrue M, Siguret V, Neuwirth M, et al. Contrast between prevalence of HIT antibodies and confirmed HIT in hospitalized COVID-19 patients: a prospective study with clinical implications. Thromb Haemost. 2020;121(07):971-975. [DOI] [PubMed] [Google Scholar]
- 34.Helms J, Tacquard C, Severac F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) . High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ionescu F, Jaiyesimi I, Petrescu I, et al. Association of anticoagulation dose and survival in hospitalized COVID-19 patients: a retrospective propensity score-weighted analysis. Eur J Haematol. 2021;106(2):165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santi RM, Sciancalepore P, Olivieri G, Contino L, Ladetto M.. The rationale for the use of unfractionated heparin (UFH) in the treatment of thromboembolic events (TE) in patients infected with COVID-19. Blood Transfus. 2020;18(SUPPL 4):S484-S485. [Google Scholar]
- 37.Warrior S, Behrens E, Gezer S, Venugopal P, Jain S.. Heparin-induced thrombocytopenia in patients with COVID-19. Blood Adv. 2020; 136(suppl1):17-18. [Google Scholar]
- 38.Madala S, Krzyzak M, Dehghani S.. Is COVID-19 an independent risk factor for heparin-induced thrombocytopenia? Cureus. 2021;13(2):e13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Julian K, Bucher D, Jain R.. Autoimmune heparin-induced thrombocytopenia: a rare manifestation of COVID-19. BMJ Case Rep. 2021;14(5):e243315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators; Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385(9):790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brodard J, Kremer Hovinga JA, Fontana P, Studt JD, Gruel Y, Greinacher A.. COVID-19 patients often show high-titer non-platelet-activating anti-PF4/heparin IgG antibodies. J Thromb Haemost. 2021;19(5):1294-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greinacher A, Warkentin TE.. Risk of heparin-induced thrombocytopenia in patients receiving thromboprophylaxis. Expert Rev Hematol. 2008;1(1):75-85. [DOI] [PubMed] [Google Scholar]
- 44.Pouplard C, May MA, Regina S, Marchand M, Fusciardi J, Gruel Y.. Changes in platelet count after cardiac surgery can effectively predict the development of pathogenic heparin-dependent antibodies. Br J Haematol. 2005;128(6):837-841. [DOI] [PubMed] [Google Scholar]
- 45.Selleng K, Warkentin TE, Greinacher A.. Heparin-induced thrombocytopenia in intensive care patients. Crit Care Med. 2007;35(4):1165-1176. [DOI] [PubMed] [Google Scholar]
- 46.Comer SP, Cullivan S, Szklanna PB, et al. ; COCOON Study investigators . COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021;19(2):e3001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldman M, Hermans C.. Thrombotic thrombocytopenia associated with COVID-19 infection or vaccination: possible paths to platelet factor 4 autoimmunity. PLoS Med. 2021;18(5):e1003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.