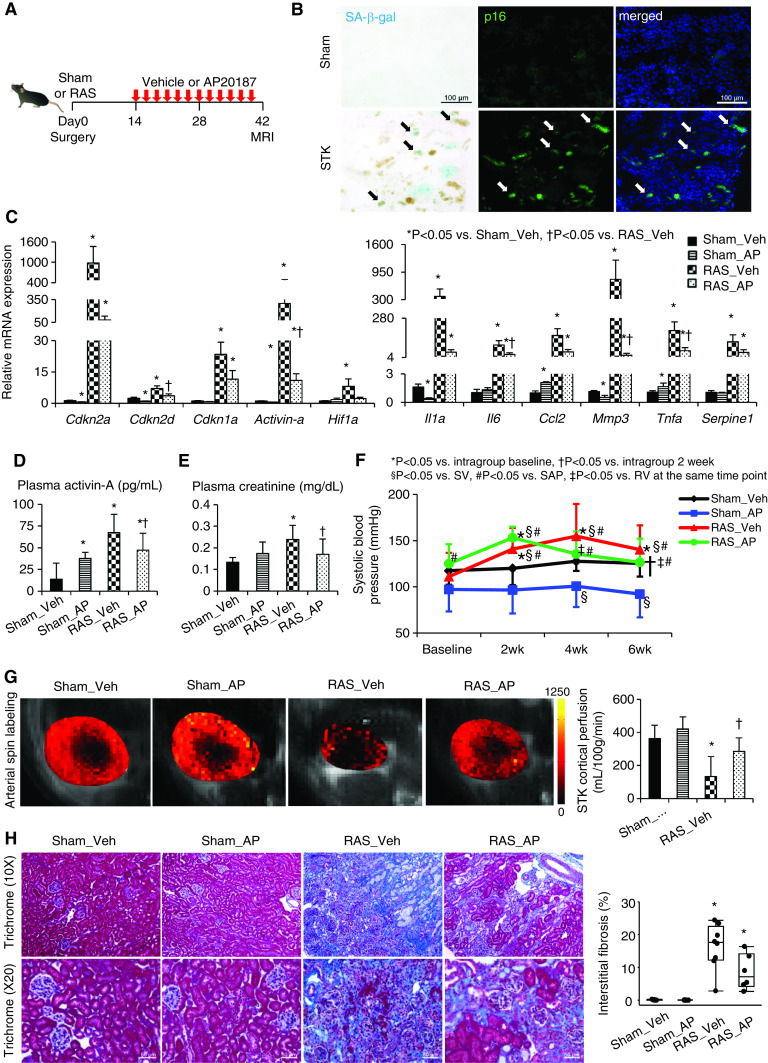
Figure 1.
Chronic ischemia induces senescence in the stenotic kidney (STK), and clearance of cells highly expressing p16Ink4a by AP improves renal function and oxygenation. (A) Experimental design in INK-ATTAC transgenic mice studied 6 weeks after sham or RAS surgeries. (B) SA-β-gal (cyan/blue) and p16 (green) colocalize (arrows) on mouse STK cryosections. Blue, 4′,6-diamidino-2-phenylindole. Renal gene expression of senescence and SASP factors using (C) real-time PCR (relative to Gapdh), (D) plasma levels of activin-A by ELISA, (E) creatinine levels using Jaffe reactions, and (F) SBP levels measured by the tail-cuff method using an automated oscillometric device in sham or RAS treated with vehicle (veh) or AP. *P<0.05 versus intragroup baseline, †P<0.05 versus intragroup after 2 weeks, §P<0.05 versus sham-vehicle group, #P<0.05 versus sham-AP group, ‡P<0.05 versus RAS-vehicle at the same time point. (G) Renal perfusion maps generated by arterial spin-labeling MRI (milliliters per 100 g per minute; brighter red, higher perfusion). (H) Masson trichrome staining and semiautomatic quantification on ten randomly chosen fields per section (using AxioVision) showed that increased STK fibrosis was slightly blunted by AP. Mean±SD or median±interquartile range (n=6–8). *P<0.05 versus sham-vehicle group, †P<0.05 versus RAS-vehicle group (t tests or Wilcoxon test).