Figure 4. FLT3 promotes mIDH2 acetylation through Y107-phosphorylation of mIDH2 that enhances ACAT1 recruitment, while, simultaneously, FLT3 inhibits SIRT3 through Y226 phosphorylation.
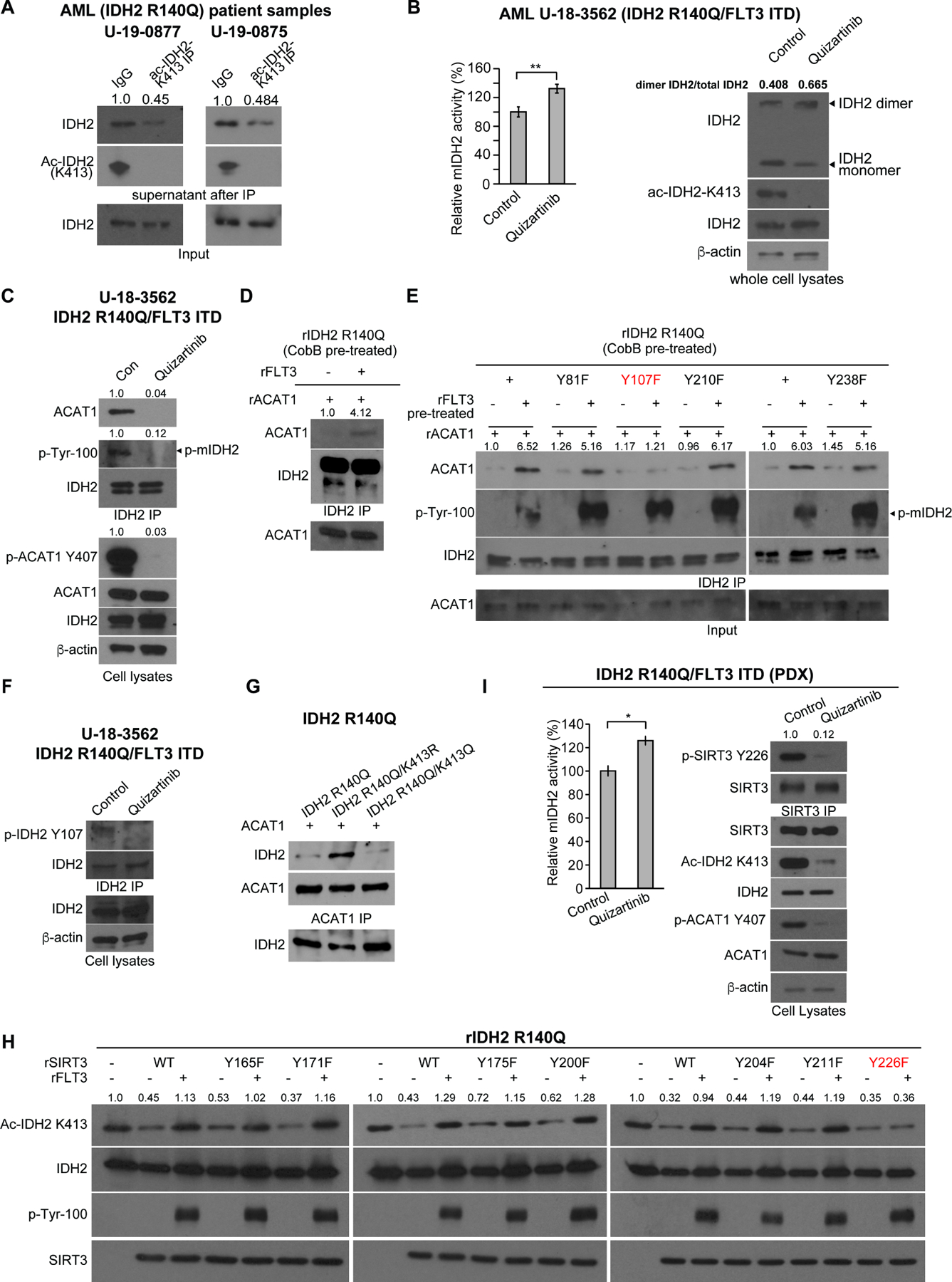
(A) Determination of stoichiometry of K413-acetylation of mutant IDH2 in human primary leukemia cells from two AML patients. K413-acetylated IDH2 protein was cleared from cell lysates by immunoprecipitation (IP) using specific acetyl-IDH2 (ac-K413) antibody, followed by Western blotting to determine protein amount of residual non-acetylated IDH2 in supernatant after IP.
(B) Human primary AML cells expressing IDH2 R140Q and FLT3 ITD mutants were treated with or without FLT3 inhibitor quizartinib, followed by mIDH2 activity assay (left) and PAGE by native gel and Western blotting to detect dimeric and monomeric IDH2, and K413-acetylation levels of IDH2 (right).
(C) Human primary AML cells expressing IDH2 R140Q and FLT3 ITD mutants were treated with or without FLT3 inhibitor quizartinib, followed by co-immunoprecipitation analysis. Binding between endogenous IDH2 and ACAT1, as well as tyrosine phosphorylation of IDH2 and ACAT1 (Y407) in human primary AML cells were detected by Western blotting.
(D) Purified rIDH2 R140Q protein was pre-treated with cobB followed by treatment with active rFLT3, prior to incubation with rACAT1. Co-immunoprecipitation (IDH2 IP) was performed followed by Western blotting to detect binding between mIDH2 and ACAT1.
(E) Purified rIDH2 R140Q variants with individual Y⭢F mutations were pre-treated with cobB followed by treatment with active rFLT3, prior to incubation with rACAT1. Coimmunoprecipitation (IDH2 IP) was performed followed by Western blotting to detect binding between mIDH2 and ACAT1.
(F) Human primary AML cells expressing IDH2 R140Q and FLT3 ITD mutants were treated with or without FLT3 inhibitor quizartinib, followed by IDH2 immunoprecipitation prior to Western blotting to detect Y107 phosphorylation level of endogenous IDH2 using a specific phosphor-IDH2 antibody (p-Y107).
(G) Purified rIDH2 R140Q variants were pre-treated with cobB followed by treatment with active rFLT3, prior to incubation with rACAT1. Co-immunoprecipitation (ACAT1 IP) was performed followed by Western blotting to detect binding between mIDH2 and ACAT1.
(H) Purified SIRT3 variants with individual Y⭢F mutations were pre-treated with rFLT3 prior to incubation with purified rIDH2 R140Q protein as a substrate in an in vitro deacetylase activity assay. K143 acetylation of IDH2 was detected by Western blotting.
(I) Human primary AML cells expressing IDH2 R140Q and FLT3 ITD mutants expanded in xenograft mice (PDX) were treated with or without FLT3 inhibitor quizartinib, followed by mIDH2 enzyme activity assay (left) and immunoprecipitation using SIRT3 antibody (right) prior to Western blotting to detect Y226 phosphorylation level of endogenous SIRT3 using a specific phosphor-SIRT3 antibody (p-Y226).
The error bars represent mean values ±SD from three replicates of each sample (*:0.01<P<0.05); Data are mean ± SD; p values were obtained by a two-tailed Student’s t-test.
