Abstract
Roylea elegans Wall. ex Benth. is a lemon-scented hoary shrub belonging to the mint family (Lamiaceae). Traditionally, a local tribe of the Himalayan region uses leaves for scabs and skin infections. The aerial parts and leaves are widely used to cure various skin ailments. The plant is well known for two furanoid diterpenes, royeleganin and royelegafuran. The aqueous extract of Roylea elegans (AERE) leaves was investigated for wound-healing effects in rats using a physically induced burn model by assessing different parameters. Animals were divided into four groups (six rats in each group). Group I animals were considered as disease control and topically given base cream. Group II was considered as standard control and treated topically with Framycetin sulphate cream (1% w/w). Group III and IV animals were treated topically with creams containing 5 or 10% of AERE, respectively. Several parameters such as wound contraction rate, epithelialization period, and enzymatic and non-enzymatic antioxidant markers along with pro- and anti-inflammatory cytokines were studied followed by histopathological studies. The animals treated with AERE cream exhibited significant declination in the wound area and increased collagen content as compared to the disease control group. The results showed that the lower dose (5%) of AERE produced a significant decrease in the epithelialization period, wound contraction rate, and collagen content. Increased levels of cytokine production may be one of the mechanisms in accelerating the wound-healing process. The study established the traditional claim as an antioxidant and wound-healing potential of Roylea elegans by promoting the accelerated wound-healing activity against the physically induced burn model.
Keywords: Collagen, Epithelialization, MPO, Pro-inflammatory cytokines, Roylea elegans, Terpenes, Wound healing
Introduction
Burns and wounds suffering are still major problems, having severe complications and involving high costs for therapy. Tissue damage from extreme heat, radioactivity, or corrosive chemicals leads to Escher (burned and traumatized tissue) which may lead to the addition of injury to nearby tissues and a major source of infection, contamination, and sepsis through bacterial growth (Jaiprakash et al. 2006).
Wound healing is a complex process in the skin characterized by re-epithelialization, granular tissue formation, and extracellular matrix remodeling. Several steps of the healing process are accomplished mainly by keratinocytes and dermal fibroblasts as well as by bioactive molecules processing several growth factors, cytokines along with their receptors, and matrix molecules (Hackam and Ford 2002; Singer and Clark 2002; Werner and Grose 2003). Apart from this, the repair process goes under the proliferation, migration, and working of fibroblasts and keratinocytes. The improvement in wound healing is reflected in enhanced cellular activities (Ahmad et al. 2013).
Different countries and cultures are managing and enhancing their efforts in the treatment of burn injuries with many topical formulations (Akhoondinasab et al. 2014). Currently, there are many synthetic topical formulations such as silver sulfadiazine available in the market for burn wounds, but their use is limited because of toxicity and negative effects on fibroblasts (Atiyeh et al. 2007; Politano et al. 2013). However, the mainstay of therapy is to increase collagen content and prevent the infections of burn wounds until the body regenerates the damaged tissues and skin (Saeedan et al. 2016). Therefore, researchers are focusing to find effective herbs and their formulations for wound healing with anti-microbial properties.
In the recent decade, several herbs and their formulations have been investigated to facilitate and accelerate the wound-healing process and regeneration of tissue (Mukherjee et al. 2003; Tam et al. 2014; Garcia-Orue et al. 2017; Sharma et al. 2021). Citrus peel extracts showed therapeutic effects in the treatment and prevention of chronic wounds especially in diabetes (Ahmad et al. 2013). Herbal-based products can treat burn wounds because of their different constituents such as flavonoids, oils, alkaloids, saponins, tannins, phenolic compounds, and terpenoids (Ahmad et al. 2013). In addition to the easy availability of herbal medicines, these have low cost, possess very few side effects, and are generally considered to be safe (Kimura et al. 2006; Stipcevic et al. 2006; Hosseini et al. 2007). Previous data reported the wound-healing action of Saffron (Crocus sativus) and green tea extract for wound healing and tissue regeneration in second-degree burn wounds (Khorasani et al. 2008; Fatemi et al. 2014).
Several medicinal plants belonging to the mint family (Lamiaceae) have been extensively reported for their wound-healing potential since ancient times against burn injuries. Roylea elegans (Lamiaceae) is commonly indigenous to Western Himalayas distributed from Kashmir to Nepal, is an erect, lemon-scented hoary shrub of the monotypic genus. The local villagers in Kumaon and Garhwal regions used leaves in scabs and skin infections (Upadhyay et al. 2017). The people of the tribal region use leaves of this plant to cure various skin ailments (Prakash and Aggarwal 2010). The aerial parts of the plant possess pentacosane, octacosanol, friedelin, beta-amyrin, beta-sitosterol, betulonic acid, and two furanoid diterpenes, i.e., royeleganin and royelegafuran. It was observed that the plant also contains a new triterpene named moronic acid (Upadhyay et al. 2011).
Although the traditional usage of Roylea elegans in wound healing is extensively documented, its benefits have yet to be scientifically verified. Therefore, the present study was conducted to investigate the wound-healing effects along with tissue regeneration of creams containing AERE in a physically induced burn model.
Materials and methods
Chemicals
Framycetin sulphate cream 1% w/w (Ranbaxy, India), ethanol, sterilized cotton, and diethyl ether were purchased from Himgiri Traders, Dehradun. All other chemicals used in the study were of analytical grade.
Plant material
Fresh leaves of Roylea elegans Wall. ex Benth. were collected from district Pithoragarh (Uttarakhand, India) and authenticated by Dr. H.B. Singh, Director, Department of Raw Material And Herbarium, NISCAIR, New Delhi, India (Ref. NISCAIR/RHMD/Consult/-2010–11/1561/159). The collected leaves were thoroughly made free from any earthy matter, dried under shade, and then powdered by using a mechanical grinder.
Preparation of aqueous extract from leaves of Roylea elegans
The 500 g powdered leaves were soaked in distilled water (1: 5 w/v) at room temperature (25 ± 1 °C). After 24 h, the supernatant was removed and again the residue was re-soaked in fresh distilled water. The process was repeated in triplicate. The extract was concentrated under a rotary evaporator. The concentrated extract was subjected to lyophilization till a dry powder was not obtained. The lyophilized powder was kept in a desiccator till further use.
Preparation and formulation of AERE creams
AERE creams were prepared at 5% and 10% extract, respectively, were mixed with liquid paraffin, cetyl alcohol, stearyl alcohol, and Span 80 at a temperature of 70 °C. The extract was heated with distilled water containing Tween 80, propyl, and methylparaben to make the aqueous phase. The aqueous and oil phases were triturated and homogenized for 15 min at 500 rpm. Allow the cream to cool to room temperature.
Preliminary phytochemical screening
AERE was investigated for the determination of preliminary phytochemical screening of various phytoconstituents such as alkaloids, flavonoids, glycosides, phenolic compounds and tannins, steroids, saponins, proteins, carbohydrates, and triterpenes, following standard methods of coloring and precipitation (Gul et al. 2017; Madike et al. 2017).
Experimental animals
Wistar albino rats (either sex) weighing 180–220 g have been procured from the animal house of Siddhartha Institute of Pharmacy, Dehradun, India. The animals were kept in an air-conditioned room with a temperature of 25 ± 1 °C, relative humidity of 55 ± 5%, and a 12 h/12 h light/dark cycle. The animals were provided hygienic conditions, a standard chow diet (Ashirwad Industries, Ropar, India), and water ad libitum.
Ethics statement
The experimental protocol was designed accordingly to ethical norms as per Institutional Animal Ethics Committee Guidelines (1435/PO/a/11/CPCSEA). All efforts were made as much as possible to reduce animal suffering.
Acute dermal toxicity study
Acute dermal toxicity study was studied as per Organisation for Economic Co-operation and Development (OECD guideline no. 402. Healthy, adult, Wistar albino rats both males and females (nulliparous and non-pregnant) weighing around 180–220 g were used in the study. The extract dose was selected as per OECD guideline 402 at one dose level was 2000 mg/kg body weight.
Animals were divided into the following groups (n = 6): Control groups (each control group of male and females) and extract-treated group (applied to each group of male and female). The hair coat was removed from the skin of animals just before 24 h of conduction of the test. The extract was applied to the shaved area dressed with porous gauze and non-irritating tape for 24 h. In control groups, porous gauze moistened with physiological saline was applied on shaved area and held in contact with non-irritating tape similar to the treatment groups. After 24 h, porous gauze was removed from successive groups, and animals were kept under observation for 14 days. The weight of individual animals was determined on the first day of application and after 14 days thereafter. Any changes in skin color, eyes, and mucous membranes, behavioral patterns, diarrhea, salivation, and tremors were observed on the last day. The mortality rate was also recorded.
Wound-healing experimental protocol
The experimental protocol was designed as an excision burn wound model to evaluate the wound-healing activity of cream containing AERE (5 and 10%). Wistar albino rats were categorized into four groups of six animals each. Group I received base cream and served as disease control (DC). Group II received standard Framycetin sulphate cream 1% w/w and served as standard control (SC). Groups III and IV received AERE cream in 5 and 10% concentrations, respectively.
Excision burn wound model
The protocol was designed and developed as per the method followed by Kimura et al. (2007). The animals were anaesthetized using Diethyl ether and the back of each rat was shaved. The naked backs were subsequently wiped with ethanol (70%) and the burn patches were made using customized soldering iron on the naked area. About 7 mm diameter with soldering iron (1 mm thickness) having temperature of 250 °C were made as burn patches.
The AERE cream (5% and 10%) and standard Framycetin sulphate cream (1% w/w) were applied topically on the affected area consequently for 21 days to their respective groups. The areas of burn wounds were determined by measuring with calipers as length × wide. The contraction rate and epithelization period were studied in this protocol.
Evaluation of granulation tissue and antioxidant levels
The wet granulation tissues were homogenized in phosphate buffer (10% w/v, pH 7.0) at 4 °C using a glass Teflon homogenizer followed by centrifugation at 12,000 rpm (30 min at 4 °C). The supernatants fluid was used for the biochemical estimation of protein levels (Krohn 2002) and oxidative stress parameters such as total glutathione (Alisik et al. 2019), superoxide dismutase (Senthilkumar et al. 2021), and catalase (Hadwan 2018). Superoxide dismutase (SOD) enzyme activity is based on the reduction of the formation of nitro blue tetrazolium NADH-phenazine methosulphate-nitro blue tetrazolium. Assessment of catalase (CAT) assay depends on the ability of an enzyme to decomposes into oxidize hydrogen peroxide (H2O2). Furthermore, total glutathione (GSH) activity was estimated by the capacity of GSH to reduce DTNB.
Estimation of myeloperoxidase (MPO)
Myeloperoxidase (inflammatory marker) was estimated using granulation tissue and homogenized (5% w/v) in hexadecyltrimethylammonium bromide (HTAB) in phosphate buffer (pH 6.0). The homogenized mixture undergoes a free thaw cycle thrice times, sonicated for 10 s, and centrifuged at 14,000 rpm for 45 min. The resulting supernatant solution is used for estimation of MPO level (Bradley et al. 1982).
Estimation of pro- and anti-inflammatory cytokine induction
On the 16th post-wounding day, blood samples were collected from all the animals of each group and stored in EDTA-containing tubes for further determination of inflammatory markers. Pro-inflammatory cytokines (IL-6 and TNF-α) and anti-inflammatory cytokines (IL-10) were determined by sandwich enzyme-linked immunosorbent assay (ELISA) using IL-6 and TNF-alpha and IL-10 (Invitrogen) ELISA kits. Assays were prepared and performed as per the manufacturer's instructions. The concentration of cytokines was determined in pg/ml by plotting the graph for standard. All the experiments were performed in triplicate to ensure the observations.
Histopathological studies
Exaggerated tissues were collected on the last day from each group and transferred to a solution of formalin (10%). The tissues were washed and embedded in paraffin wax (mp 58–60 °C). The tissue sections were cut and stained with hematoxylin followed by eosin stain for collagen synthesis and its morphological changes. The photomicrographs of the tissue sections were captured using a charge-coupled device camera under a light microscope (Olympus BX51TF; Olympus Corporation, Tokyo, Japan) at × 400 magnification power (Ansari et al. 2021).
Statistical analysis
The wound area and wound breaking strength between groups at different time intervals were compared using one-way ANOVA, followed by Dunnett’s test. Values are represented as Mean ± SEM. Values p < 0.05 were considered statistically significant.
Results
Preliminary phytochemical screening
The percentage yield of AERE was found to be 10.09%. The phytochemical analysis of AERE was performed based on preliminary screening chemical tests of various phytoconstituents as mentioned in Table 1. The analysis revealed the presence of carbohydrates, alkaloids, tannins, terpenes, and saponins.
Table 1.
Preliminary phytochemical screening of aqueous extract of Roylea elegans (AERE) leaves
| S. No. | Phytoconstituents | Chemical tests | Inference |
|---|---|---|---|
| 1 | Carbohydrates |
Molisch test Fehling’s test Benedict’s test Barfoed’s test |
+ |
| 2 | Alkaloids |
Dragendorff test Mayer’s test Wagner’s test Hager’s test |
+ |
| 3 | Flavonoids | Shinoda test | + |
| 4 | Terpenes | 2–3 granules of tin metal + 2 ml thionyl chloride solution + 1 ml extract = pink color | + |
| 5 | Steroids | Salkowski test | + |
| 6 | Saponins | Foam test | + |
| 7 | Tannins | Braymer’s Test | + |
| 8 | Glycosides |
Legal test Baljet test Modified Borntrager’s test Keller Killiani test |
+ |
| 9 | Anthraquinones | Borntrager’s test | − |
+ present, − absent
Acute dermal toxicity
The toxic effects of AERE were observed. No poisonous signs or mortality were seen in animals.
Wound-healing activity
The creams containing AERE were administered topically at doses of 5% and 10%, to the test groups and the rats observed for 21 days for signs of abnormal behavioral disabilities and mortality. The results showed that the animals treated with both doses of AERE (5 and 10%) produced a statistically significant (p < 0.05) improvement in wound healing in comparison to the disease control group and results were almost comparable with the standard treatment group. The data showing body weight, body temperature, and daily food intake of different groups are shown in Table 2.
Table 2.
Effect of cream containing aqueous extract of Roylea elegans (AERE) leaves on physical parameters against physically induced burn model in rats
| Groups | Body weight (g) | Body temperature (F) | Daily food intake (%) |
|---|---|---|---|
| Disease control | 152.50 ± 0.81 | 101.00 ± 0.35 | 72.50 ± 1.07 |
| Standard control | 155.33 ± 1.01 | 100.83 ± 0.29 | 78.33 ± 2.01 |
| AERE (5%) | 163.17 ± 1.92** | 96.67 ± 0.40**# | 92.50 ± 1.07**# |
| AERE (10%) | 165.67 ± 2.24** | 97.50 ± 0.21** | 87.50 ± 2.69** |
All values are expressed as mean ± SEM (n = 6). Mean difference is significant at the **p < 0.01 level compared to disease control group and #p < 0.05 compared to standard control. Statistical analysis was done by one-way analysis of variance (ANOVA) followed by Dunnett’s test
Excision wound contraction and epithelialization
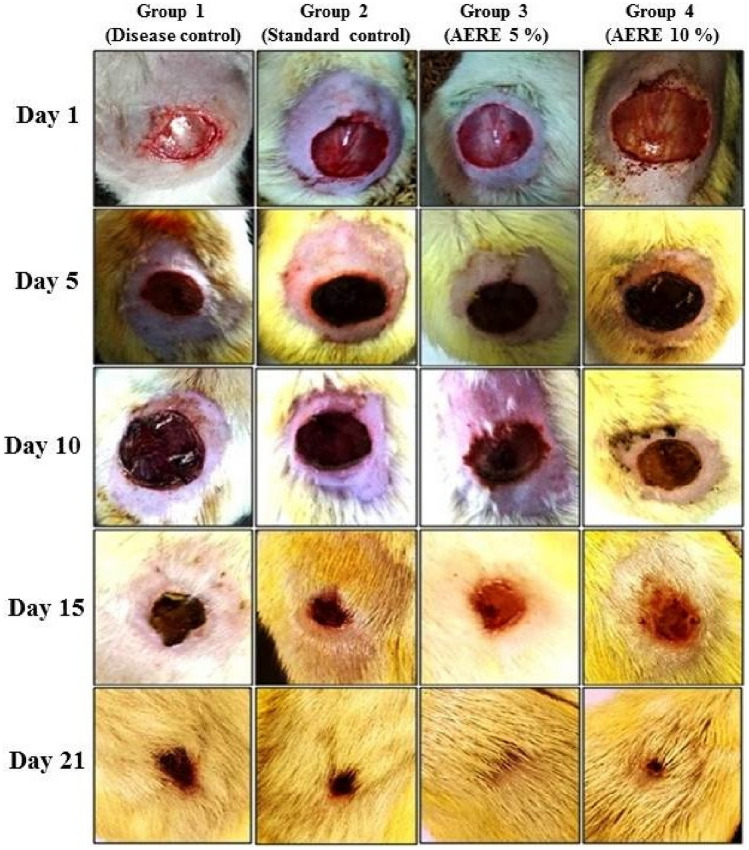
The excision wound contraction was found to be significantly improved in groups treated with AERE cream as compared to disease and standard control groups. The low dose of 5% AERE cream shows a significant (p < 0.05) increase in wound contraction (99.96%), formation of collagen, and tissue re-epithelialization as compared to disease control and standard group (Table 3 and Fig. 1).
Table 3.
Effect of cream containing aqueous extract of Roylea elegans (AERE) leaves on wound contraction and epithelialization of excision wound in physically induced burn model in rats
| Groups | Wound area (cm) and % wound contraction | Epithelialization Period (days) | |||
|---|---|---|---|---|---|
| Day 5 | Day 10 | Day 15 | Day 21 | ||
| Disease Control |
2.92 ± 0.07 (19.55%) |
2.48 ± 0.07 (45.68%) |
1.79 ± 0.07 (65.53%) |
0.99 ± 0.09 (82.45%) |
19.50 ± 1.45 |
| Standard Control |
2.86 ± 0.06 (28.46%) |
2.39 ± 0.05 (51.18%) |
1.56 ± 0.05 (72.25%) |
0.65 ± 0.09 (91.39%)** |
16.25 ± 1.30 |
| AERE (5%) |
2.52 ± 0.05 (24.34%)** |
2.16 ± 0.03 (68.57%)** |
1.33 ± 0.04 (87.48%)** |
0.05 ± 0.02 (99.96%)**# |
13.80 ± 1.00**# |
| AERE (10%) |
2.60 ± 0.06 (26.00%)** |
2.23 ± 0.05 (62.45%)**# |
1.50 ± 0.03 (83.21%)**# |
0.10 ± 0.03 (96.35%)** |
15.15 ± 0.80**# |
All values are expressed as mean ± SEM (n = 6). Mean difference is significant at the *p < 0.05, **p < 0.01 level compared to disease control group and #p < 0.05 compared to standard control group. Statistical analysis was done by one-way analysis of variance (ANOVA) followed by Dunnett’s test
Fig. 1.
Effect of cream containing aqueous extract of Roylea elegans (AERE) leaves on excision wound contraction and tissue epithelization
Determination of antioxidants and myeloperoxidase activities in wet granulation tissue
The results are tabulated in Table 4. AERE showed a significant increase in protein levels, antioxidants (GSH, SOD, and CAT), and a decrease in MPO levels. Protein levels were found to be significantly increased at a low dose (58.35 ± 2.09, p < 0.01) of AERE when compared with the disease control group (33.98 ± 2.48). GSH, SOD, and CAT levels were significantly increased in the AERE-treated group at low dose (29.33 ± 1.03, 0.81 ± 0.05, and 163.67 ± 3.80) and high dose (27.41 ± 1.67, 0.71 ± 0.03, and 103.67 ± 2.12), when compared with disease control group (21.53 ± 1.46, 0.43 ± 0.02, and 47.83 ± 1.76). In animals treated with AERE cream, MPO levels were found to be decreased at low dose (16.35 ± 0.41, p < 0.01) and high dose (19.82 ± 0.57, p < 0.05), respectively, when compared with the disease control group (22.83 ± 1.35).
Table 4.
Effect of cream containing aqueous extract of Roylea elegans (AERE) leaves on wet granulation tissue weight, antioxidants, and myeloperoxidase (MPO) levels against physically induced burn model in rats
| Groups | Wet tissue (mg/100 g bw) |
Protein (mg/g tissue) |
GSH (nmole/mg protein) |
SOD (Units/mg protein) |
CAT (Units/mg protein) |
MPO (Units/mg wet tissue) |
|---|---|---|---|---|---|---|
| Disease Control | 271.81 ± 13.50 | 33.98 ± 2.48 | 21.53 ± 1.46 | 0.43 ± 0.02 | 47.83 ± 1.76 | 22.83 ± 1.35 |
| Standard Control | 405.17 ± 19.87** | 63.33 ± 1.28** | 30.33 ± 1.23** | 0.87 ± 0.06** | 170.50 ± 2.56** | 13.75 ± 0.31** |
| AERE (5%) | 383.50 ± 23.34** | 58.35 ± 2.09** | 29.33 ± 1.03** | 0.81 ± 0.05** | 163.67 ± 3.80** | 16.35 ± 0.41** |
| AERE (10%) | 302.03 ± 11.57 | 40.51 ± 1.87 | 27.41 ± 1.67* | 0.71 ± 0.03** | 103.67 ± 2.12** | 19.82 ± 0.57* |
All values are expressed as mean ± SEM (n = 6). Mean difference is significant at the *p < 0.05, **p < 0.01 level compared to disease control group. Statistical analysis was done by one-way analysis of variance (ANOVA) followed by Dunnett’s test
Determination of pro-inflammatory and anti-inflammatory cytokine production
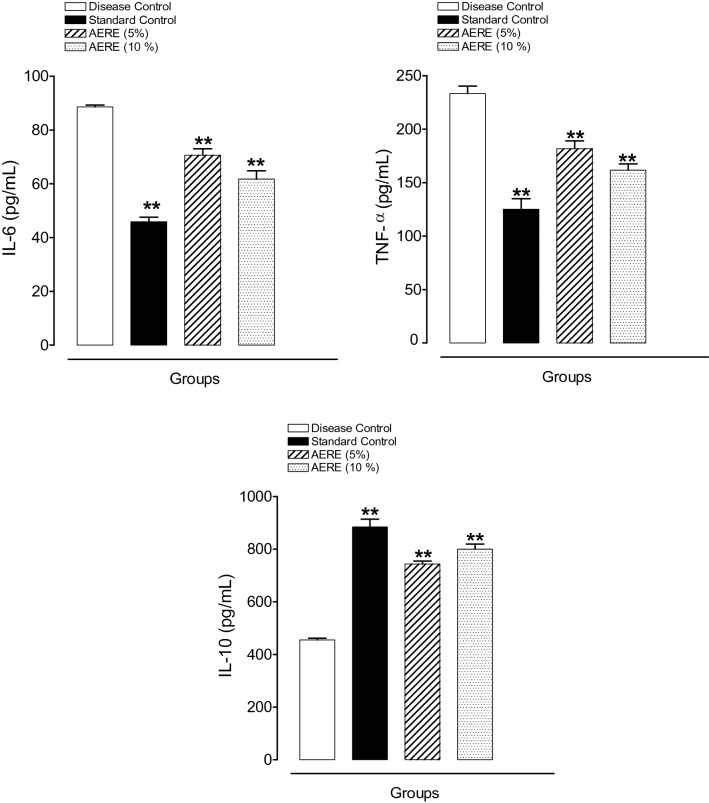
The results are shown in Fig. 2. The production of Pro-inflammatory cytokines (IL-6 and TNF-α) was found to be significantly (p < 0.01) lower in the AERE-treated group at low dose (70.64 ± 2.34, and 181.67 ± 7.37) and high dose (61.75 ± 3.09, and 161.67 ± 5.86), respectively, than that in the disease control group (88.56 ± 0.73, and 233.33 ± 6.90).
Fig. 2.
Effect of cream containing aqueous extract of Roylea elegans (AERE) leaves on release of pro-inflammatory (IL-6 and TNF-α) and anti-inflammatory (IL-10) markers against physically induced burn model in rats. Data expressed as mean ± SEM (n = 6). Mean difference was significant at the **p < 0.01 level compared to disease control group. Statistical analysis was done by one-way analysis of variance (ANOVA) followed by Dunnett’s test
The level of IL-10 in the AERE-treated group was found to be significantly (p < 0.01) higher at low dose (743.17 ± 11.43) and high dose (799.83 ± 19.32) when compared with the disease control group (454.83 ± 6.90).
Histopathological analysis
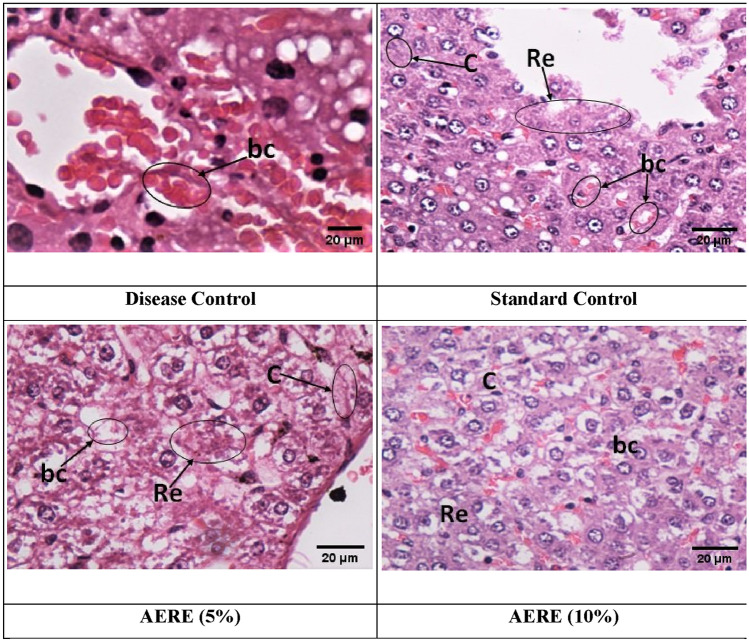
The histopathological studies of tissues from the disease control group showed a complete loss of superficial epithelium cells with inflammation as shown in Fig. 3. The standard control group and treated groups showed reduced inflammation and increased levels of fibroblast cells, collagen fibers, and capillary blood. Moreover, Group III administered with a low dose of AERE cream (5%) showed more epithelialization with the access amount of extracellular matrix synthesis and new blood vessel formation.
Fig. 3.
Effect of cream containing aqueous extract of Roylea elegans (AERE) leaves on histopathological studies. Photomicrographs of normal control group showing loss of superficial epithelium cells with inflammation however RME and Framycetin sulphate cream (standard control) treated groups showed increase in the levels of fibroblast cells, collagen fibers and blood capillaries (re re-epithelialization, c collagen, bc blood capillaries)
Discussion
No sign of any illness and mortality was reported during and after the completion of the toxicity study, hence the selected dose was agreed upon for pharmacological study. Burn injury is a dynamic process that promotes immune dysfunction and inflammation characterized by vascular endothelial growth factor (VEGF) (Chen et al. 2017). The VEGF enhances the vascular permeability responsible for increased migration and proliferation of endothelial cells. Therefore, it plays a vital role in tissue repair during wound healing and angiogenesis which increases vascular permeability (Bao et al. 2009).
Furthermore, during the healing process, formation of collagen stimulates homeostasis and re-epithelialization provides granulation tissue formation in wound contraction. Hence, the present study is focused on physically induced burn wound healing by granulation, collagenation, and VEGF stimulated by natural compounds and their derivatives. Terpenes are an important class of chemicals found in the mint family that help to rebuild epidermal cells and control gene expression patterns to decrease inflammation. They generally show by regulating the VEGF signaling pathway and suppress the VEGFR-controlled PI3K/Akt/mTOR signaling pathway. Therefore, the antiangiogenic effect by various terpenes (mono, di, or sesqui) may responsible for controlling the wound by regulating the VEGF in re-epithelialization (Parveen et al. 2019).
In the inflammatory phase of wound healing, pro-inflammatory cytokines (TNF-α and IL-6) are known to play a major role in enhancing angiogenesis (Pattanayaka and Sunitab 2008). Our study revealed that the level of TNF-α and IL-6 declined in groups treated with AERE cream. Previous literature reported that TNF-α inhibits collagen formation and hydroxyproline production which is essential for the final part of the proliferative phase in wound healing, but the low level of TNF-α and IL-6 did not interfere with collagen formation and hydroxyproline production (Siqueira et al. 2010).
In the present study, AERE cream significantly elevated the level of IL-10 production, an anti-inflammatory cytokine produced by various cells including macrophages and T-lymphocytes. Wound healing is widely recognized to be linked to a reduction in pro-inflammatory cytokines. IL-10 production influences the wound-healing mechanism by reducing the pro-inflammatory/profibrotic mediators and inflammatory cells to the wound area (Ribbons et al. 1997). Furthermore, higher IL-10 levels may be linked to a reduction in TNF-cell production, resulting in the wound-healing process (Bodger et al. 1997). IL-10 acts as a protective action in tissue damage caused by inflammation. Therefore, elevation in IL-10 levels can regulate TNF-α production in macrophages leading to increased wound healing.
Treatment of wounds with AERE cream results in increased IL-10 concentration and downregulation of TNF-α and IL-6. Findings from the present study suggested that AERE regulates anti-inflammatory and pro-inflammatory cytokines.
Synthesis of collagen in wound area by providing the strength and re-epithelialization promotes maximum wound-healing process. Many plant products such as flavonoids, alkaloids, terpenes, polyphenolic compounds accelerated the synthesis of collagen and possibly even support the cross-linkages as the collagen matures (Shukla et al. 1999). The possible wound-healing process in a burn by the plant might be due to the presence of alkaloids and a small concentration of diterpenes. The 21 days study in the burn model significantly revealed that the healing process was better in AERE-treated groups at both concentrations (5% and 10%) as compared to the group treated with standard cream.
The observed results were significant and confirmed by the increased fibroblast cells, collagen fibers and blood capillaries in the extract-treated group and re-epithelialization was also maximum. Histopathological studies also confirm the maximum healing process in AERE-treated group. As a result, it was discovered that the traditional use of the plant's leaves for different skin illnesses was based on its physical wound-healing mechanism against burns.
Conclusion
The physically induced burn wound-healing action of AERE shows the significant healing process at both doses along with standard. The effect may be attributed to the phytoconstituents present in the extract which may be either due to their individual or cumulative effect. The % wound contraction and re-epithelialization were found to be higher at the low concentration of AERE cream as compared to standard cream which reveals the traditional action of Roylea elegans in the burn wound model.
Author contributions
GU collected the leaves of the plant. GU and HM prepared an aqueous extract of leaves. Wound-healing activity was performed by GU, NT, RJ, and JU. All authors contributed in the writing of the manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ahmad M, Ansari MN, Alam A, Khan TH. Oral dose of citrus peel extracts promotes wound repair in diabetic rats. Pakistan J Biol Sci. 2013;16(20):1086–1094. doi: 10.3923/pjbs.2013.1086.1094. [DOI] [PubMed] [Google Scholar]
- Akhoondinasab MR, Akhoondinasab M, Saberi M. Comparison of healing effect of Aloe vera extract and silver sulfadiazine in burn injuries in experimental rat model. World J Plast Surg. 2014;3:29–34. [PMC free article] [PubMed] [Google Scholar]
- Alisik M, Neselioglu S, Erel O. A colorimetric method to measure oxidized, reduced and total glutathione levels in erythrocytes. J Lab Med. 2019;43(5):269–277. doi: 10.1515/labmed-2019-0098. [DOI] [Google Scholar]
- Ansari MN, Rehman NU, Karim A, Imam F, Hamad AM. Protective effect of Thymus serrulatus essential oil on cadmium-induced nephrotoxicity in rats, through suppression of oxidative stress and downregulation of NF- κB, iNOS, and Smad2 mRNA expression. Molecules. 2021;26(5):1252. doi: 10.3390/molecules26051252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33:139–148. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153(2):347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodger K, Wyatt JI, Heatley RV. Gastric mucosal secretion of interleukin-10: relations to histopathology, helicobacter pylori status, and tumour necrosis factor-alpha secretion. Gut. 1997;40(6):739–744. doi: 10.1136/gut.40.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi MJ, Nikoomaram B, Rahimi AA, Talayi D, Taghavi S, Ghavami Y. Effect of green tea on the second degree burn wounds in rats. Indian J Plast Surg. 2014;47:370–374. doi: 10.4103/0970-0358.146593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Orue I, Gainza G, Gutierrez FB, Aguirre JJ, Evora C, Pedraz JL, Hernandez RM, Delgado A, Igartua M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int J Pharm. 2017;523(2):556–566. doi: 10.1016/j.ijpharm.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci World J. 2017;2017:1–7. doi: 10.1155/2017/5873648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam DJ, Ford HR. Cellular biochemical and clinical aspects of wound healing. Surg Infect (Larchmt) 2002;3:S23–S35. doi: 10.1089/sur.2002.3.s1-23. [DOI] [PubMed] [Google Scholar]
- Hadwan MH. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018;19:7. doi: 10.1186/s12858-018-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SV, Tanideh N, Kohanteb J, Ghodrati Z, Mehrabani D, Yarmohammadi H. Comparison between Alpha and silver sulfadiazine ointments in treatment of Pseudomonas infections in 3rd degree burns. Int J Surg. 2007;5:23–26. doi: 10.1016/j.ijsu.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Jaiprakash B, Chandramohan B, Reddy DN. Burn wound healing activity of Euphorbia hirta. Anc Sci Life. 2006;25:16–18. [PMC free article] [PubMed] [Google Scholar]
- Khorasani G, Hosseinimehr SJ, Zamani P, Ghasemi M, Ahmadi A. The effect of saffron (Crocus sativus) extract for healing of second-degree burn wounds in rats. Keio J Med. 2008;57:190–195. doi: 10.2302/kjm.57.190. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Sumiyoshi M, Kawahira K, Sakanaka M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br J Pharmacol. 2006;148:860–870. doi: 10.1038/sj.bjp.0706794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Sumiyoshi M, Sakanaka M. Effects of Astilbe thunbergii rhizomes on wound healing: part 1. Isolation of promotional effectors from Astilbe thunbergii rhizomes on burn wound healing. J Ethnopharmacol. 2007;109(1):72–77. doi: 10.1016/j.jep.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Krohn RI. The colorimetric detection and quantitation of total protein. Curr Protoc Cell Biol. 2002;15:A.3H.1–A.3H.28. doi: 10.1002/0471143030.cba03hs15. [DOI] [PubMed] [Google Scholar]
- Madike LN, Takaidza S, Pillay M. Preliminary phytochemical screening of crude extracts from the leaves, stems, and roots of Tulbaghia violacea. Int J Pharmacogn Phytochem Res. 2017;9(10):1300–1308. doi: 10.25258/phyto.v9i10.10453. [DOI] [Google Scholar]
- Mukherjee PK, Mukherjee K, Rajesh Kumar M, Pal M, Saha BP. Evaluation of wound healing activity of some herbal formulations. Phytother Res. 2003;17(3):265–268. doi: 10.1002/ptr.931. [DOI] [PubMed] [Google Scholar]
- Parveen A, Subedi L, Kim HW, Khan Z, Zahra Z, Farooqi MQ, Kim SY. Phytochemicals targeting VEGF and VEGF-related multifactors as anticancer therapy. J Clin Med. 2019;8:350. doi: 10.3390/jcm8030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayaka SP, Sunitab P. Wound healing, anti-microbial and antioxidant potential of Dendrophthoe falcata (L.f) Ettingsh. J Ethnopharmacol. 2008;120(2):241–247. doi: 10.1016/j.jep.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Politano AD, Campbell KT, Rosenberger LH, Sawyer RG. Use of silver in the prevention and treatment of infections: silver review. Surg Infect (larchmt) 2013;14(1):8–20. doi: 10.1089/sur.2011.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash V, Aggarwal A. Traditional uses of ethanomedicinal plants of lower foot hills of Himanchal Pradesh-I. Indian J Traditional Knowledge. 2010;9:519–521. [Google Scholar]
- Ribbons KA, Thompson JH, Liu X, Pennline K, Clark DA, Miller MJ. Anti-inflammatory properties of interleukin-10 administration in hapten-induced colitis. Eur J Pharmacol. 1997;323(2–3):245–254. doi: 10.1016/s0014-2999(97)00017-4. [DOI] [PubMed] [Google Scholar]
- Saeedan AS, Gabr GA, Soliman GA, Fayed MH, Ansari MN. The potential anti-inflammatory and wound healing activities of chitosan in rats. Adv Biores. 2016;7(6):1–7. doi: 10.15515/abr.0976-4585.7.6.17. [DOI] [Google Scholar]
- Senthilkumar M, Amaresan N, Sankaranarayanan A. Plant-microbe interactions. New York: Springer Protocols Handbooks; 2021. Estimation of superoxide dismutase (SOD) pp. 117–118. [Google Scholar]
- Sharma A, Khanna S, Kaur G, Singh I. Medicinal plants and their components for wound healing applications. Future J Pharm Sci. 2021;7:53. doi: 10.1186/s43094-021-00202-w. [DOI] [Google Scholar]
- Shukla A, Rasik AM, Jain GK, Shankar R, Kulshrestha DK, Dhawan BN. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J Ethnopharmacol. 1999;65:1–11. doi: 10.1016/s0378-8741(98)00141-x. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RAF. Mechanism of disease—cutaneous wound healing. N Engl J Med. 2002;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Siqueira MF, Li J, Chehab L, Desta T, Chino T, Krothpali N, Behl Y, Alikhani M, Yang J, Braasch C, et al. Impaired wound healing in mouse models of diabetes is mediated by tnf-alpha dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1) Diabetologia. 2010;53:378–388. doi: 10.1007/s00125-009-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipcevic T, Piljac A, Piljac G. Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns. 2006;32:24–34. doi: 10.1016/j.burns.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam JC, Ko CH, Lau KM, To MH, Kwok HF, Chan YW, Siu WS, Etienne-Selloum N, Lau CP, Chan WY, et al. A Chinese 2-herb formula (NF3) promotes hindlimb ischemia-induced neovascularization and wound healing of diabetic rats. J Diabetes Complicat. 2014;28(4):436–447. doi: 10.1016/j.jdiacomp.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Upadhyay G, Malik J, Joshi R, Lakshmayya Singh UK. Hepatoprotective potential of lyophilized hydro-alcoholic extract of Roylea elegans wall. Against CCL4 and PCM induced hepatotoxicity in Wistar rats. Ann Pharmacol Pharm. 2017;2(4):1045. [Google Scholar]
- Upadhyay G, Kamboj P, Malik J. Pharmacognostical studies and evaluation of quality parameters of Roylea elegans Wall. (aerial parts) J Pharm Pharm Sci. 2011;2:1678–1685. [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]