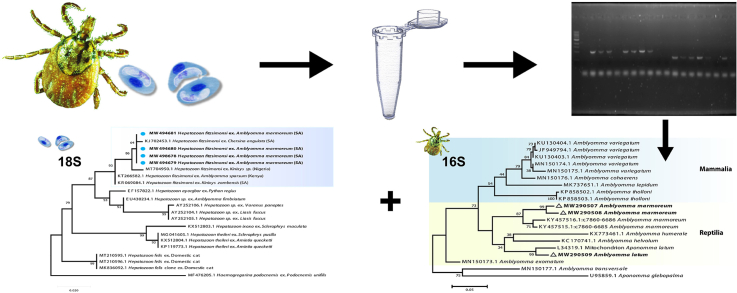
Abstract
In South Africa, the role of reptilian ticks in the transmission of haemoparasites is lacking, in part, due to limited information on tick diversity and their associated haemoparasites. The aim of this research was to identify tick species parasitizing reptiles and to molecularly screen these ectoparasites for species of the blood apicomplexan genus Hepatozoon. Samples were collected from Ndumo Game Reserve, KwaZulu-Natal, and the Cape Columbine region, Western Cape. Reptiles collected included 2 snakes, 2 monitor lizards of a single species respectively, as well as 17 tortoises of four species. Ticks collected from these were morphologically identified as Amblyomma latum (n = 2) and Amblyomma marmoreum (n = 98), this identification was molecularly confirmed using 16S rRNA and CO1 genes. Screening for Hepatozoon was done by amplifying the 18S rRNA gene. A species of Hepatozoon, Hepatozoon fitzsimonsi, was identified from A. marmoreum ticks, with an overall prevalence of 10%. This Hepatozoon species, has been described parasitizing tortoises from southern Africa, and has been reported from ticks infesting tortoises from Kenya, East Africa. Even though ticks have been suggested to be the likely vector of this Hepatozoon species, with this supported by the findings of Hepatozoon-like developmental stages in ticks collected off of infected tortoises, a recent systematic revision placed this species in a newly erected genus Bartazoon, a genus vectorised by biting insects. The present study thus provides further support for ticks acting as the potential vectors of H. fitzsimonsi.
Keywords: Amblyomma, Hepatozoon, PCR, Morphology, Tortoises
Graphical abstract
Highlights
-
•
Two tick species Amblyomma marmoreum and A. latum infesting South African reptiles.
-
•
DNA of tortoise haemogregarine Hepatozoon fitzsimonsi detected from unengorged A. marmoreum.
-
•
Suggestive of a long-lived infection of this parasite in these acarines.
-
•
Further support for ticks as potential vectors of H. fitzsimonsi.
1. Introduction
Members of family Ixodidae Dugès, 1834 are divided into two groups (Prostriata and Metastriata) that are distinctive in their morphology, physiology, phylogenetic and other characteristics (Hornok et al., 2020). They are the most significant vectors of emerging zoonotic pathogens, which can be severe and life-threatening to humans; second to mosquitoes in the number of human diseases they transmit (Kho et al., 2015; Luz et al., 2018). A number of tick species have formed a close relationship with their vertebrate hosts, with a marked preference for reptiles and amphibians. According to studies of valid names, Amblyomma Koch, 1844 was documented as the second largest group of Ixodidae ticks with 130 valid species distributed worldwide, and nine of these species, including three species that belonged to the former genus Aponomma Neumann, 1899, have been reported in South Africa (Walker, 1991; Barker and Murrell, 2004; Horak et al., 2006; Guglielmone et al., 2014).
Species of Hepatozoon Miller, 1908 are frequently observed as intraerythrocytic or intraleucocytic haemoparasites of reptiles (Smith, 1996; Telford, 2009). These adeleid haemogregarines display a heteroxenous life cycle requiring a vertebrate intermediate host and an invertebrate vector or definitive host (Smith, 1996; Telford, 2009; Karadjian et al., 2015). The systematics and diversity of species of apicomplexans such as Hepatozoon are still poorly understood, the genus Hepatozoon itself being paraphyletic based on estimated relationships using sequences of the 18S rRNA gene (see Barta et al., 2012, Haklová-Kočíková et al., 2014; Kvičerová et al., 2014, Cook et al., 2016). In an attempt to aid in the resolution of this, a new genus Bartazoon Karadjian et al., 2015 was erected, this genus included all species of reptiles, previously assigned to Hepatozoon. One of the characteristics of the genus Bartazoon is that the vectors of all members are biting insects, whilst species of Hepatozoon have ticks and mites as vectors (Karadjian et al., 2015). To date, the genus Bartazoon has not been widely accepted as the monophyly of this genus is not well supported (Maia et al., 2016; Borges-Nojosa et al., 2017). Furthermore, the taxonomic revision which established Bartazoon did so based on the use of a single gene – the 18S rRNA gene. As such, the taxonomic changes associated with this new genus was considered premature (Maia et al., 2016; Borges-Nojosa et al., 2017; Cook et al., 2018). Therefore, as suggested and recommended by Maia et al. (2016) and Borges-Nojosa et al. (2017), and the recent phylogenetic findings of Hrazdilová et al. (2021) opposing Bartazoon, we will conservatively continue to refer to species parasitizing reptilian hosts as species of Hepatozoon and not Bartazoon. In South Africa, species of Hepatozoon infecting reptiles have been described from four species of lizards, two species of snake and five species of tortoise (Cook et al., 2018). That in the tortoises includes only a single species Hepatozoon fitzsimonsi (Dias, 1953) (Cook et al., 2014, 2018). This species of haemogregarine was originally described as belonging to Haemogregarina (sensu stricto) by Siddall (1995) but subsequently reassigned to Hepatozoon based on a study into the morphology of its peripheral blood stages, findings of potential life cycle stages in Amblyomma ticks found feeding on tortoises, as well as phylogenetic analysis of the 18S rDNA sequence fragments from microscopically-positive tortoise blood (Cook et al., 2014).
Despite the importance of Amblyomma ticks from a one-health perspective, knowledge on their taxonomic status, potential of reptiles as their hosts, and the pathogens they may transmit, is limited (Sánchez-Montes et al., 2019; Mendoza-Roldan et al., 2021). In this sense, the current study aimed to identify tick species parasitizing reptiles and to molecularly screen these ectoparasites for species of the blood apicomplexan genus Hepatozoon. Furthermore, this study provides molecular findings of H. fitzsimonsi in species of Amblyomma, with some comments on the potential of these acarine parasites as potential hosts for this haemogregarine.
2. Materials and methods
2.1. Sample collection and identification
Adult ticks (n = 100) were collected from reptiles (tortoises, snakes and monitor lizards) in the Cape Columbine region, Western Cape (WC) (17° 51′ 56″ E 32° 49′ 2″ S) (2011) and the Ndumo Game Reserve, KwaZulu-Natal (KZN) (32°18′49″E; 26°54′33″S) (2014–2017), thereafter they were allowed the opportunity to digest their blood meals for approximately 10–20 days (Desser et al., 1995; Smith, 1996), and subsequently stored in 70% ethanol until further analysis. Sampling was carried out under the relevant permits (WC: 0035-AAA004-00383; KZN: OP 839/2014, OP 4374/2015, OP 4092/2016, OP 4264/2017), and received the relevant ethical approval (reference numbers: 920203595 and NWU-00372-16-A5 respectively). Morphological identification to species level was done with the aid of a Nikon AZ100M microscope and guidelines for tick identification as provided by Theiler (1945) and Theiler and Salisbury (1959). Representative tick samples from the study area were also used for molecular studies.
2.2. DNA extraction from tick specimens
Whole individual tick specimens were used for DNA extraction following the standard protocol method for animal tissue as detailed in the DNeasy Tissue Kit (Qiagen, Germany). Following filtration, the filtrate was collected and stored at −20 °C.
2.3. PCR amplification and phylogenetic analysis
DNA samples extracted from the ticks were used as a template for PCR amplification. For the identification of ticks, a 710bp long fragment of the cytochrome c oxidase subunit I (CO1) gene was amplified in a conventional PCR with the primer pair HCO2198 (5′-GGTCAACAAATCATAAAGATATTGG-3′ and LCO1490 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) Folmer et al. (1994). Another PCR was used to amplify an approximately 460bp long fragment of the 16S rDNA gene of Ixodidae (Black and Piesman, 1994), with the primers 16S + 1 (5′-CTGCTCAATGATTTTTTAAATTGCTGTGG-3′) and 16S-1 (5′-CCGGTCTGAACTCAGATCAAG T-3′). For the screening of Hepatozoon spp., PCR was conducted using P1 (5′-CACAGGGAGGTAGTGACAAG-3′) and P2 (5′-AAGAATTTCACCTATGACAG-3′) primers that amplified 430bp of the hypervariable region of 18S rRNA gene of piroplasms (Schnittger et al., 2004). The primer set HAMF 5′-GCCAGTAGTCAT-ATGCTTGTC-3’ (Criado-Fornelio et al., 2006) and HepR900 5′-CAAATCTAAGAATTTCACCTCTGAC-3' (Ujvari et al., 2004) were also used for screening of Hepatozoon spp. However, none of the sequences amplified by this latter set were of good quality.
Conditions for PCR of all genes were as follows: initial denaturation at 95 °C for 3 min, followed by 35 cycles, entailing a 95 °C denaturation for 30 s, annealing at 58 °C for 30 s with an end extension at 72 °C for 2 min, and following the cycles a final extension of 72 °C for 10 min (Netherlands et al., 2018).
All PCR reactions were performed with volumes of 25 μl, using 12.5 μl Thermo Scientific DreamTaq PCR master mix (2×) (2×DreamTaq buffer, 0.4 mM of each dNTP, and 4 mM MgCI2), 1.25 μl of each primer (10 μM), and 2 μl of DNA. Double distilled water (ddH2O) was used to make up final reaction volume. Reactions were undertaken in a Bio-Rad C1000 Touch™ Thermal Cycler PCR machine (Bio-Rad, Hemel Hempstead, UK). An agarose gel (1%) stained with gel red was used to visualise resulting amplicons under UV light.
Positive PCR products from each sample were sent to a commercial sequencing company (Inqaba Biotechnical Industries (Pty) Ltd, Pretoria, South Africa) for purification and sequencing in both directions. Sequence and species identity were determined using the Basic Local Alignment Search Tool (BLAST) (http://blast.ncbi.nlm.nih.gov/). Sequences obtained in the current study were deposited in the NCBI GenBank database under the following accession numbers: [GenBank: MW290507–MW290509 (16S rRNA); MW513957–MW513958 (CO1)] for ticks and [GenBank: MW494678–MW494681] for Hepatozoon. For phylogenetic analysis, comparative sequences of Hepatozoon species were downloaded from GenBank and aligned to the sequences generated within this study. Sequences were aligned using the Clustal W alignment tool under the default settings and implemented in MEGA Ver 7.1. To infer phylogenetic relationships Maximum likelihood (ML) method was used. Prior to the analyses a model test was performed to determine the most suitable nucleotide substitution model, according to the Akaike information criterion (AIC) using jModelTest 2.1.7 (Guindon and Gascuel, 2003; Darriba et al., 2012).
3. Results
3.1. Morphological analysis of Amblyomma ticks
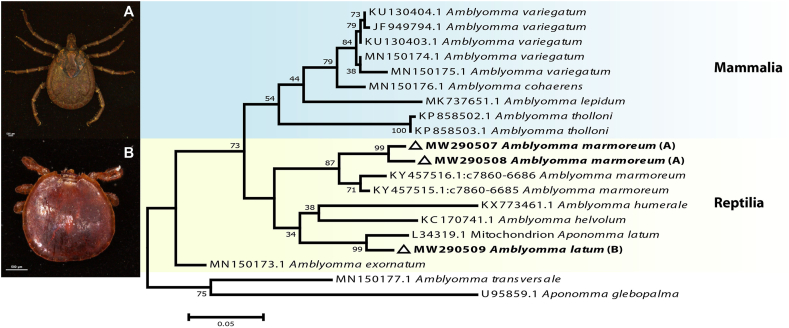
Ninety-eight (98) adult ticks were identified as Amblyomma marmoreum and two (2) as Amblyomma latum based on their morphology. The A. marmoreum was found infesting tortoises (n = 5 from the Cape, n = 9 from Ndumo), as well as two monitor lizards (both Varanus albigularis) from Ndumo, while A. latum was found on one of the two snakes (both Naja mossombica) (Table 1). The characteristics of the adult A. latum and adult A. marmoreum are as described by Theiler (1945) and Theiler and Salisbury (1959), respectively (Fig. 1).
Table 1.
Ticks of the genus Amblyomma collected from reptiles in South Africa during this study.
| Tick species | Number | Host species |
|---|---|---|
| A. latum | 2 | Naja mossambica (2) |
| A. marmoreum (male) | 54 | Varanus albigularis (2), Stigmochelys pardalis (25), Chersina angulata (8), Kinixys spp. (7), K. zombensis (12) |
| A. marmoreum (female) | 44 | V. albigularis (2), S. pardalis (16), K. zombensis (13), C. angulata (7), Kinixys spp. (6). |
Number of ticks collected from hosts is indicated next to each host.
Fig. 1.
Maximum likelihood analysis of Amblyomma tick species based on the 16S rRNA sequences. Bootstrap values at the major nodes are of percentage agreement among 1000 replicates. The branch scale represents substitutions per site.
3.2. Molecular analysis of Amblyomma ticks and their Hepatozoon spp.
The PCR analysis of the DNA isolates indicates amplification of 460bp for the 16S rRNA gene and ∼700bp for the CO1 gene. BLASTn results for both mtDNA genes matched with the expected species, although notable divergence levels were observed in both cases (Fig. 1). The CO1 gene was not able to amplify the A. latum. Based on the 16S rRNA phylogenetic analysis, the A. marmoreum from the current study constitutes a monophyletic clade (99% bootstrap value) closely affiliated to the same lineage group of A. marmoreum, while A. latum from this study formed a monophyletic clade with A. latum with 99% bootstrap value. Both A. marmoreum and A. latum formed a monophyletic group with other Amblyomma species of reptiles, clearly distinct from other Amblyomma species of mammals with a >70% bootstrap value (Fig. 2).
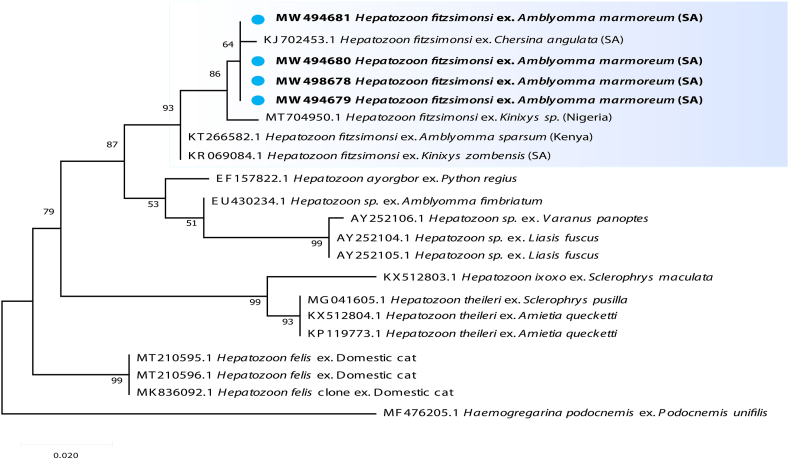
Fig. 2.
Maximum likelihood analysis of species of Hepatozoon based on the 18S rRNA sequences. Bootstrap values at the major nodes are of percentage agreement among 1000 replicates. The branch scale represents substitutions per site.
The overall prevalence of Hepatozoon was 10/100 (10%). It was detected from 1/4 (25%) A. marmoreum parasitizing Varanus albigularis and 9/94 (10%) A. marmoreum parasitizing tortoises. No Hepatozoon was detected from A. latum. The BLASTn analysis for the 430bp 18S rRNA of the Hepatozoon sequences had a 99% similarity with H. fitzsimonsi (KR069084.1) from South African tortoise Kinixys zombensis, and clustered in a single clade with H. fitzsimonsi from another South African tortoise, a tortoise collected from Nigeria and a tick collected from a tortoise in Kenya (Fig. 2).
4. Discussion
Members of the genus Amblyomma are known as hosts and vectors of various pathogenic diseases that can cause considerable economic losses in tropical and sub-tropical regions of the world (Ogo et al., 2012, 2017; Vesco et al., 2011; Hornok et al., 2020). According to data encompassing several surveys, A. marmoreum is most frequently encountered on tortoises, as observed in this study (Theiler, 1959; Horak et al., 2006). On the other hand, A. latum is known to infest snakes with occasional records from lizards and even in some mammals, which represent incidental infestations (Walker, 1991; Theiler, 1945); the present study observed this tick species from a snake (Naja mossambica).
Even though the CO1 gene did not amplify A. latum DNA, both phenotypic characteristics and the 16S rRNA confirmed this species as A. latum. Based on the analysis of both genes in the current study, the phylogenetic trees showed a clustering of A. marmoreum tick sequences into two groups with high bootstrap values, indicating that the possibility of having a diverse population of A. marmoreum ticks in South Africa cannot be ruled out. However, a more detailed phylogenetic study of A. marmoreum needs to be undertaken to confirm any intraspecific variation. Ogo et al. (2017) found similar results in Nigeria based on the characterization of 16S rRNA of A. variegatum.
However, the current study was limited by the lack of A. marmoreum and A. latum sequences in GenBank, as it did not afford us the opportunity to draw inferences. Nevertheless, it demonstrates the abundance and population diversity of Amblyomma ticks infesting some reptiles in South Africa, thereby raising the potential for the existence of various tick-borne zoonoses, possibly beyond those that are already documented in the country.
Hepatozoon fitzsimonsi was reassigned from the genus Haemogregarina (sensu lato) to the genus Hepatozoon (Cook et al., 2014), and more recently to genus Bartazoon (Karadjian et al., 2015). The aforementioned authors suggested ticks as probable vectors for H. fitzsimonsi due to the sporogonic stages (including sporocysts and sporozoites) observed in A. marmoreum and Amblyomma sylvaticum found infesting H. fitzsimonsi infected and seemingly uninfected tortoises. An attempt to molecularly identify and compare these stages to H. fitzsimonsi in the peripheral blood of infected tortoises was made with only bands of the targeted size obtained. Unfortunately, the sequences obtained in the Cook et al. (2014) study were unsatisfactory. To the best of our knowledge, there has been no molecular report to date showing the definite presence of H. fitzsimonsi in ticks from South Africa. As highlighted by Karadjian et al. (2015), there is a high potential for error when analysing vectors morphologically and molecularly for haemogregarine infections. It is possible to wrongly assume that the sporogonic stages observed in a vector such as the tick collected from a haemogregarine infected vertebrate, such as in the case of H. fitzsimonsi from tortoises, are the same parasite. This is particularly problematic if the ticks collected were engorged at the time of collection and molecular analysis.
In the present study, H. fitzsimonsi was detected from A. marmoreum infesting tortoises and monitor lizards. The presence of H. fitzsimonsi in ticks found infesting monitor lizards is noteworthy, as no infections of H. fitzsimonsi have been recorded in these reptiles (Cook et al., 2016). From southern African monitor lizards, two species of Hepatozoon have been described, Hepatozoon camari (Dias, 1954) and Hepatozoon varani (Laveran, 1905) from Mozambique and South Africa respectively (Dias, 1954; Laveran, 1905 respectively; Cook et al., 2016). However, morphologically, these species do not conform to H. fitzsimonsi. Hepatozoon fitzsimonsi displays a mature gamont measuring 17.2 × 3.8 μm, which is banana shaped with a rounded to square compact nucleus (Cook et al., 2014). In comparison, H. camari measures 11.8 × 5 μm (banana form) or 14.3 × 18.3 μm (curved form), with an irregular nucleus; whilst H. varani measures 14 × 3 μm, the gamont folded (suggesting a length of 24 μm), the nucleus occurring on the fold. Hepatozoon fitzsimonsi gamonts were never seen to fold (Cook et al., 2009, 2014). The occurrence of a tick harbouring H. fitzsimonsi collected from a monitor lizard could be considered as a good example for the potential errors highlighted by Karadjian et al. (2015), assuming the parasite sequenced in the ticks is related to that found in the vertebrate, when effectively the ‘vectors’ are still engorged with a previous blood meal from a different host. To avoid this scenario, this study only used ticks which had been given the opportunity to digest their blood meals. This would then suggest that the infection is long-lived in the tick, with the potential of ticks acting as hosts and potential vectors increasing in possibility.
In Kenya, during a study aimed at the molecular detection of tick-borne pathogens (TBPs), Amblyomma sparsum and Amblyomma falsomarmoreum, collected from tortoises, were documented to harbour H. fitzsimonsi (Omondi et al., 2017). Similarly, this study only used unengorged ticks for the same reasoning as mentioned above – that is to minimise contamination from the vertebrate hosts’ DNA and effectively that of peripheral blood infections. Notably, H. fitzsimonsi was sequenced from ticks collected from only tortoises, the sequence data showing a high prevelance of H. fitzsimonsi in these ticks. Furthermore, A. falsomarmoreum was found exclusively on tortoises and is a tick species specifically associated with these vertebrates. Again, this leads to the question of whether or not H. fitzsimonsi is long-lived in ticks and is perhaps undergoing sporogonic development within these acarines. However, this will only be determined once more complete life cycle data is achieved for this haemogregarine, but simultaneously, the coincidence of the above findings is difficult to ignore.
5. Conclusion
As H. fitzsimonsi has been observed infecting several species of tortoises from southern Africa, it would appear to be a generalist parasite with a potentially wide-distribution (Dias, 1953; Cook et al., 2009, 2014). Taking into account the results of the present study and that of Omondi et al. (2017), as well as the findings of Cook et al. (2014), it cannot be ruled out at this stage that ticks may play a role as vector in the life cycle of H. fitzsimonsi. If these acarines are found to act as vectors for this haemogregarine, this may pose a conservation risk to naïve hosts, particularly those that are critically endangered. It is therefore important to elucidate the routes and modes of infection of this species of Hepatozoon, particularly in light of the translocation and illegal trade in many tortoise species (see Burridge, 2001). Furthermore, if this haemogregarine is found to have ticks as vectors, it will not fit within the present characteristic confines defining the genus Bartazoon, resulting in yet another paraphyletic genus.
Declaration of competing interest
All authors contributed in the draft of this manuscript and declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Ezemvelo KZN Wildlife, South Africa, is thanked for research permits OP 839/2014, OP 4374/2015, OP 4092/2016, OP 4264/2017, as well as Cape-Nature for research permit 0035-AAA004-00383. We also thank Dr Edward Netherlands for assisting in the collection of samples from Ndumo Game Reserve. Mr W. Landman is thanked for assisting with the microscope. The first author (LSM) was funded by the National Research Foundation (NRF) – The Foundational Biodiversity Information Programme (FBIP) Scholarship (Grant number: 128335). The NRF also provided funding to CAC (NRF project CSRP190414430265, grant 120395; NRF incentive RA161107208698, grant 120237). Opinions, findings and conclusions or recommendations arrived at are those of the authors and not necessarily those of the funding bodies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This is contribution No 559 from the NWU-Water Research Group.
References
- Barker S.C., Murrell A. Systematics and evolution of ticks with a list of valid genus and species names. Parasitology. 2004;129(S1):S15. doi: 10.1017/s0031182004005207. [DOI] [PubMed] [Google Scholar]
- Black W.C., Piesman J. Phylogeny of hard-and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. Unit. States Am. 1994;91(21):10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta J.R., Ogedengbe J.D., Martin D.S., Smith T.G. Phylogenetic position of the adeleorinid coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rDNA sequences. J. Eukaryot. Microbiol. 2012;59(2):171–180. doi: 10.1111/j.1550-7408.2011.00607.x. [DOI] [PubMed] [Google Scholar]
- Borges-Nojosa D.M., Borges-Leite M.J., Maia J.P., Zanchi-Silva D., da Rocha Braga R., Harris D.J. A new species of Hepatozoon Miller, 1908 (Apicomplexa: Adelerina) from the snake Philodryas nattereri Steindachner (Squamata: Dipsadidae) in northeastern Brazil. Syst. Parasitol. 2017;94(1):65–72. doi: 10.1007/s11230-016-9676-2. [DOI] [PubMed] [Google Scholar]
- Burridge M.J. Ticks (Acari: Ixodidae) spread by the international trade in reptiles and their potential roles in dissemination of diseases. Bull. Entomol. Res. 2001;91:3–23. [PubMed] [Google Scholar]
- Cook C.A., Smit N.J., Davies A.J. A redescription of Haemogregarina fitzsimonsi Dias, 1953 and some comments on Haemogregarina parvula Dias, 1953 (Adeleorina: Haemogregarinidae) from southern African tortoises (Cryptodira: Testudinidae), with new host data and distribution records. Folia Parasitol. 2009;56(3):173–179. doi: 10.14411/fp.2009.021. [DOI] [PubMed] [Google Scholar]
- Cook C.A., Lawton S.P., Davies A.J., Smit N.J. Reassignment of the land tortoise haemogregarine Haemogregarina fitzsimonsi Dias 1953 (Adeleorina: Haemogregarinidae) to the genus Hepatozoon Miller 1908 (Adeleorina: Hepatozoidae) based on parasite morphology, life cycle and phylogenetic analysis of 18S rDNA sequence fragments. Parasitology. 2014;141(12):1611. doi: 10.1017/S003118201400081X. [DOI] [PubMed] [Google Scholar]
- Cook C.A., Netherlands E.C., Smit N.J. Redescription, molecular characterisation and taxonomic re-evaluation of a unique African monitor lizard haemogregarine Karyolysus paradoxa (Dias, 1954) n. comb. (Karyolysidae) Parasites Vectors. 2016;9(1):1–13. doi: 10.1186/s13071-016-1600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C.A., Netherlands E.C., Smit N.J., Van As J. Two new species of Hepatozoon (Apicomplexa: Hepatozoidae) parasitising species of Philothamnus (Ophidia: Colubridae) from South Africa. Folia Parasitol. 2018;65:4–11. doi: 10.14411/fp.2018.004. [DOI] [PubMed] [Google Scholar]
- Criado-Fornelio A., Ruas J.L., Casado N., Farias N.A.R., Soares M.P., Müller G., Brum J.G.W., Berne M.E.A., Buling-Saraña A., Barba-Carretero J.C. New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. J. Parasitol. 2006;92(1):93–99. doi: 10.1645/GE-464R.1. [DOI] [PubMed] [Google Scholar]
- Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012;9(8) doi: 10.1038/nmeth.2109. 772–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desser S.S., Hong H., Martin D.S. The life history, ultra-structure, and experimental transmission of Hepatozoon catesbianae n. comb., an apicomplexan parasite of the bullfrog, Rana catesbeiana and the mosquito, Culex territans in Algonquin Park, Ontario. J. Parasitol. 1995;81:212–222. [PubMed] [Google Scholar]
- Dias J.A.T.S. Subsídios para o estudo dos hematozoários dos répteis de Moçambique. Bol. Soc. Est. Moçamb. 1953;23:41–73. [Google Scholar]
- Dias J.A.T.S. Subsídios para o estudo dos hematozoários dos répteis de Moçambique 3: Duas novas espécies de Haemogregarina parasitas do Varanus albigularis albigularis (Daudin, 1802) Bol. Soc. Est. Moç. 1954;87:71–79. [Google Scholar]
- Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mel. Marine. Biol. Biot. 1994;3:294–299. [PubMed] [Google Scholar]
- Guglielmone A.A., Robbins R.G., Apanaskevich D.A., Petney T.N., Estrada-Peña A., Horak I.G. Springer; Dordrecht: 2014. The Hard Ticks of the World; pp. 978–994. Doi 10. [Google Scholar]
- Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52(5):696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Haklová-Kočíková B., Hižňanová A., Majláth I., Račka K., Harris D.J., Földvári G., Tryjanowski P., Kokošová N., Malčeková B., Majláthová V. Morphological and molecular characterization of Karyolysus–a neglected but common parasite infecting some European lizards. Parasites Vectors. 2014;7(1):1–12. doi: 10.1186/s13071-014-0555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak I.G., McKay I.J., Henen B.T., Heyne H., Hofmeyr M.D., De Villiers A.L. Parasites of domestic and wild animals in South Africa. XLVII. Ticks of tortoises and other reptiles. Onderstepoort J. Vet. Res. 2006;73(3):215–227. doi: 10.4102/ojvr.v73i3.148. [DOI] [PubMed] [Google Scholar]
- Hornok S., Kontschán J., Takács N., Chaber A.L., Halajian A., Abichu G., Kamani J., Szekeres S., Plantard O. Molecular phylogeny of Amblyomma exornatum and Amblyomma transversale, with reinstatement of the genus Africaniella (Acari: Ixodidae) for the latter. Ticks Tick-borne Dis. 2020;11(6) doi: 10.1016/j.ttbdis.2020.101494. [DOI] [PubMed] [Google Scholar]
- Hrazdilová K., Červená B., Blanvillain C., Foronda P., Modrý D. Quest for the type species of the genus Hepatozoon–phylogenetic position of hemogregarines of rats and consequences for taxonomy. Syst. Biodivers. 2021:1–10. [Google Scholar]
- Karadjian G., Chavatte J.M., Landau I. Systematic revision of the adeleid haemogregarines, with creation of Bartazoon ng, reassignment of Hepatozoon argantis Garnham, 1954 to Hemolivia, and molecular data on Hemolivia stellata. Parasite. 2015;22:31. doi: 10.1051/parasite/2015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho K.L., Koh F.X., Tay S.T. Molecular evidence of potential novel spotted fever group rickettsiae, Anaplasma and Ehrlichia species in Amblyomma ticks parasitizing wild snakes. Parasites Vectors. 2015;8(1):1–5. doi: 10.1186/s13071-015-0719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvičerová J., Hypša V., Dvořáková N., Mikulíček P., Jandzik D., Gardner M.G., Javanbakht H., Tiar G., Široký P. Hemolivia and Hepatozoon: haemogregarines with tangled evolutionary relationships. Protist. 2014;165(5):688–700. doi: 10.1016/j.protis.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Laveran A. Sur une haemogregarine de Varanus niloticus. CR Seances Soc. Biol. 1905;59:175–176. [Google Scholar]
- Luz H.R., Silva-Santos E., Costa-Campos C.E., Acosta I., Martins T.F., Muñoz-Leal S., McIntosh D., Faccini J.L.H., Labruna M.B. Detection of Rickettsia spp. in ticks parasitizing toads (Rhinella marina) in the northern Brazilian Amazon. Exp. Appl. Acarol. 2018;75(3):309–318. doi: 10.1007/s10493-018-0270-y. [DOI] [PubMed] [Google Scholar]
- Maia J.P., Harris D.J., Carranza S., Gomez-Diaz E. Assessing the diversity, host-specificity and infection patterns of apicomplexan parasites in reptiles from Oman, Arabia. Parasitology. 2016;143(13):1730–1747. doi: 10.1017/S0031182016001372. [DOI] [PubMed] [Google Scholar]
- Mendoza-Roldan J.A., Mendoza-Roldan M.A., Otranto D. Reptile vector-borne diseases of zoonotic concern. Int. J. Parasitol.: Parasites Wildl. 2021;15:132–142. doi: 10.1016/j.ijppaw.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherlands E.C., Cook C.A., Du Preez L.H., Vanhove M.P., Brendonck L., Smit N.J. Monophyly of the species of Hepatozoon (Adeleorina: Hepatozoidae) parasitizing (African) anurans, with the description of three new species from hyperoliid frogs in South Africa. Parasitology. 2018;145(8):1039–1050. doi: 10.1017/S003118201700213X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogo N.I., de Mera I.G.F., Galindo R.C., Okubanjo O.O., Inuwa H.M., Agbede R.I., Torina A., Alongi A., Vicente J., Gortázar C., de la Fuente J. Molecular identification of tick-borne pathogens in Nigerian ticks. Vet. Parasitol. 2012;187(3–4):572–577. doi: 10.1016/j.vetpar.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Ogo N.I., Okubanjo O.O., Inuwa H.M., Agbede R.I.S. Morphological and molecular characterization of Amblyomma variegatum (Acari: Ixodidae) ticks from Nigeria. Niger. Vet. J. 2017;38(3):260–267. [Google Scholar]
- Omondi D., Masiga D.K., Fielding B.C., Kariuki E., Ajamma Y.U., Mwamuye M.M., Ouso D.O., Villinger J. Molecular detection of tick-borne pathogen diversities in ticks from livestock and reptiles along the shores and adjacent islands of Lake Victoria and lake Baringo, Kenya. Front. Vet. Sci. 2017;4:73. doi: 10.3389/fvets.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Montes S., Isaak-Delgado A.B., Guzmán-Cornejo C., Rendón-Franco E., Muñoz-García C.I., Bermúdez S., Morales-Diaz J., Cruz-Romero A., Romero-Salas D., Dzul-Rosado K., Lugo-Caballero C. Rickettsia species in ticks that parasitize amphibians and reptiles: novel report from Mexico and review of the worldwide record. Ticks Tick-borne Dis. 2019;10(5):987–994. doi: 10.1016/j.ttbdis.2019.04.013. [DOI] [PubMed] [Google Scholar]
- Schnittger L., Yin H., Qi B., Gubbels M.J., Beyer D., Niemann S., Jongejan F., Ahmed J.S. Simultaneous detection and differentiation of Theileria and Babesia parasites infecting small ruminants by reverse line blotting. Parasitol. Res. 2004;92(3):189–196. doi: 10.1007/s00436-003-0980-9. [DOI] [PubMed] [Google Scholar]
- Siddall M.E. Phylogeny of adeleid blood parasites with a partial systematic revision of the haemogregarine complex. J. Eukaryot. Microbiol. 1995;42:116–125. doi: 10.1111/j.1550-7408.1995.tb01551.x. [DOI] [PubMed] [Google Scholar]
- Smith T.G. The genus Hepatozoon (Apicomplexa: Adeleina) J. Parasitol. 1996;82(4):565–585. [PubMed] [Google Scholar]
- Telford S.R. CRC Press; New York: 2009. Hemoparasites of the Reptilia: Color Atlas and Text. [Google Scholar]
- Theiler G. 1945. Ticks in the South African Zoological Survey Collection. Part III. The Ornate Aponommas. [PubMed] [Google Scholar]
- Theiler G., Salisbury L.E. 1959. Ticks in the South African Zoological Survey Collection-Part IX-The Amblyomma Marmoreum Group. [Google Scholar]
- Ujvari B., Madsen T., Olsson M. High prevalence of Hepatozoon spp. (Apicomplexa, Hepatozoidae) infection in water pythons (Liasis fuscus) from tropical Australia. J. Parasitol. 2004;90(3):670–672. doi: 10.1645/GE-204R. [DOI] [PubMed] [Google Scholar]
- Vesco U., Knap N., Labruna M.B., Avšič-Županc T., Estrada-Peña A., Guglielmone A.A., Bechara G.H., Gueye A., Lakos A., Grindatto A., Conte V. An integrated database on ticks and tick-borne zoonoses in the tropics and subtropics with special reference to developing and emerging countries. Exp. Appl. Acarol. 2011;54(1):65–83. doi: 10.1007/s10493-010-9414-4. [DOI] [PubMed] [Google Scholar]
- Walker J.B. A review of the ixodid ticks (Acari: Ixodidae) occurring in southern Africa. Onderstepoort J. Vet. Res. 1991;58:81–105. [PubMed] [Google Scholar]