Figure 1.
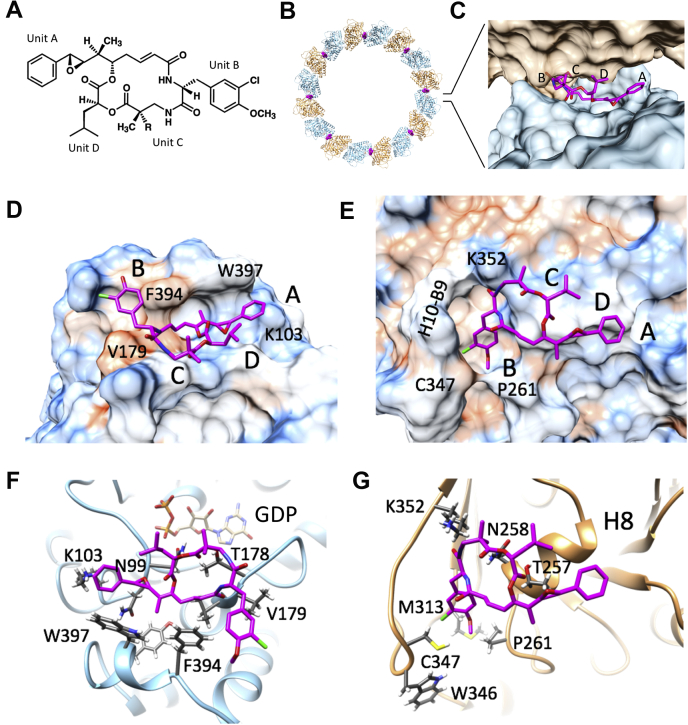
Binding site of Cp-52 on HeLa tubulin.A, structure of Cp with the units A, B, C, and D indicated. In Cp-1, there is a single methyl instead of a gem-dimethyl group in unit C (i.e., R = H in Cp-1, CH3 in Cp-52). B, tubulin ring with 8-fold symmetry induced by Cp-52; α-tubulin (brown), β-tubulin (blue), and Cp-52 (magenta). C, view of Cp-52 (stick, magenta and heteroatom) bound at the maytansine site between β-tubulin (blue, surface) and α-tubulin (brown, surface) as viewed from outside the ring. D, Cp-52 (stick, magenta and heteroatom) interaction with β-tubulin (hydrophobicity surface). E, Cp-52 (stick, magenta and heteroatom) interaction with α-tubulin (hydrophobicity surface). F, Cp-52 (stick, magenta and heteroatom) interaction with β-tubulin (ribbon, blue), as viewed toward the minus-end. GDP is indicated. G, Cp-52 (stick, magenta and heteroatom) interaction with α-tubulin (ribbon, brown), as viewed toward the plus-end. Helix H8 is indicated. Cp-52, cryptophycin-52.