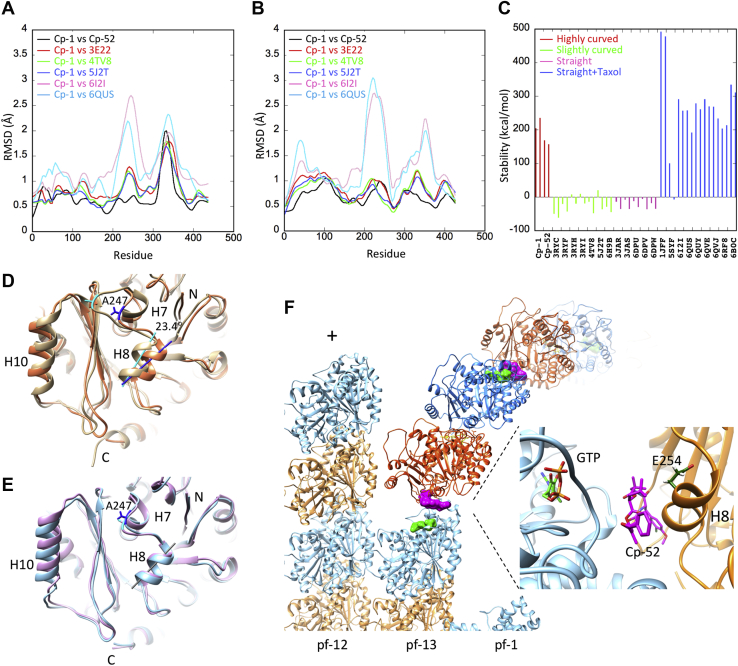
Figure 3.
Conformational changes in α-tubulin and β-tubulin upon Cp-52 binding and ring formation.A, per-residue Cα-RMSD between α-tubulin subunits in complexes with different degrees of curvature: Cp-1-bound and Cp-52-bound (black) tubulins, Cp-1-bound and colchicine–soblidotin–stathmin-bound (red) tubulins, Cp-1-bound and maytansine–stathmin-bound (green) tubulins, Cp-1-bound and vinblastine–stathmin-bound (blue) tubulins, and Cp-1-bound and Taxol-bound (pink and cyan) tubulins. Cp-1-bound and Cp-52-bound tubulin is highly curved, colchicine–soblidotin–stathmin-bound tubulin, maytansine–stathmin-bound tubulin, and vinblastine–stathmin-bound tubulin is slightly curved, and Taxol-bound tubulin is not curved. Plot curve smoothing is 10%. B, per-residue Cα-RMSD between β-tubulin subunits in complexes with different degrees of curvature. Complexes are otherwise as in (A). C, stabilities of subunits in highly curved Cp-1-bound and Cp-52-bound rings (red), slightly curved complexes (green), microtubules (magenta), and Taxol-bound microtubules (blue). The stabilities of α-tubulin and β-tubulin are shown for each structure, left and right columns, respectively. Stability values are calculated with FoldX. D, conformational differences between HeLa α-tubulin in a microtubule (Protein Data Bank ID: 6I2I, tan) and HeLa α-tubulin in a Cp-52-bound ring (brown). α:H8 (residues 252–259) in the ring is rotated 23.4° clockwise relative to that in the microtubule, as viewed toward the protofilament plus-end. The T7 loop at α:A247 is also retracted 7 Å. E, conformational differences between HeLa α-tubulin in Cp-1-bound (lilac) and Cp-52-bound (blue) rings. The structures are essentially identical, including at α:H8 (the two helix axes are coincident) and the T7 loop but differ at α:H10 where that of Cp-52 is shifted 2.4 Å toward the exterior side of the subunit. F, model of Cp-52 mechanism. Plus-end of a microtubule with a 13-3 B-lattice viewed at the seam from the lumen. Microtubule (α-tubulin, brown; β-tubulin, blue), incoming tubulin dimer (α-tubulin, orange; β-tubulin, dodger blue), Cp-52 (magenta), and GTP (green). Note, the nucleotide in our structures has GDP at the exchangeable site (see Experimental procedures section), but on a microtubule in a cell, this would be GTP. Only select ligands are shown, for clarity. Cp-52, cryptophycin-52.