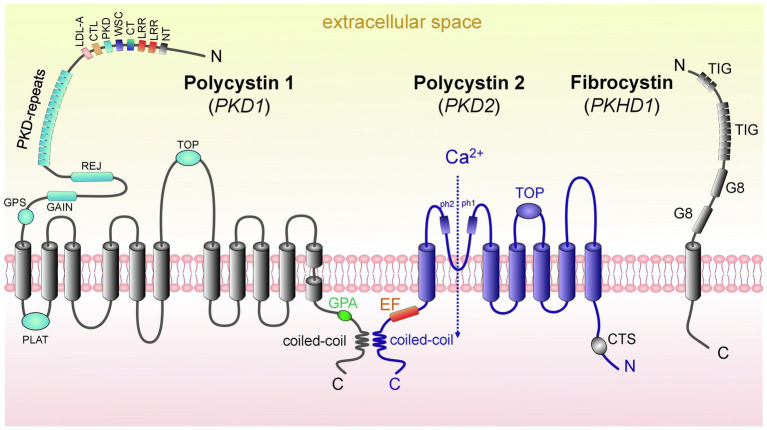
Figure 2.
Domain structure of proteins causing ARPKD and ADPKD: polycystin 1, polycystin 2, and fibrocystin. (left) PC1 contains 11 transmembrane domains (TD); in addition, there are the following domains: PC1 lipoxygenase α-toxin (PLAT) domain, tetragonal opening for polycystins domain (TOP), an intracellular C-terminus with a coiled-coil domain and a G-protein activating site (GPA), an extracellular N-terminus with a G-protein site (GPS), a G protein-coupled receptors autoproteolysis-inducing (GAIN) domain, receptor for egg jelly (REJ), PKD-related repeats, low-density lipoprotein-A domain (LDL-A), C-type lectin domain (CTL), cell wall integrity and stress component domain (WSC), C-terminal cysteine-rich domain (CT), leucine-rich repeat (LRR), and N-terminal cysteine-rich domain (NT). (middle) PC2 has 6 TD, a tetragonal opening for polycystins domain (TOP) and two pore helices (ph1 and ph2); both of its C-and N-termini are cytosolic. Of note, its C-terminus contains an EF-hand domain and a coiled-coil domain, and in the N-tail contains a ciliary targeting sequence (CTS). PC1 and PC2 can interact via their coiled-coil domains. (right) FPC contains 1 TD, a short cytosolic C-tail, and a long extracellular N-terminus. The N-terminus contains two G8 domains, which can be involved in ligand binding and catalysis, and multiple copies of an Ig-like domain (TIG). Abbreviations: Polycystin 1 (PC1), polycystin 2 (PC2), transmembrane domain (TD), fibrocystin (FPC).