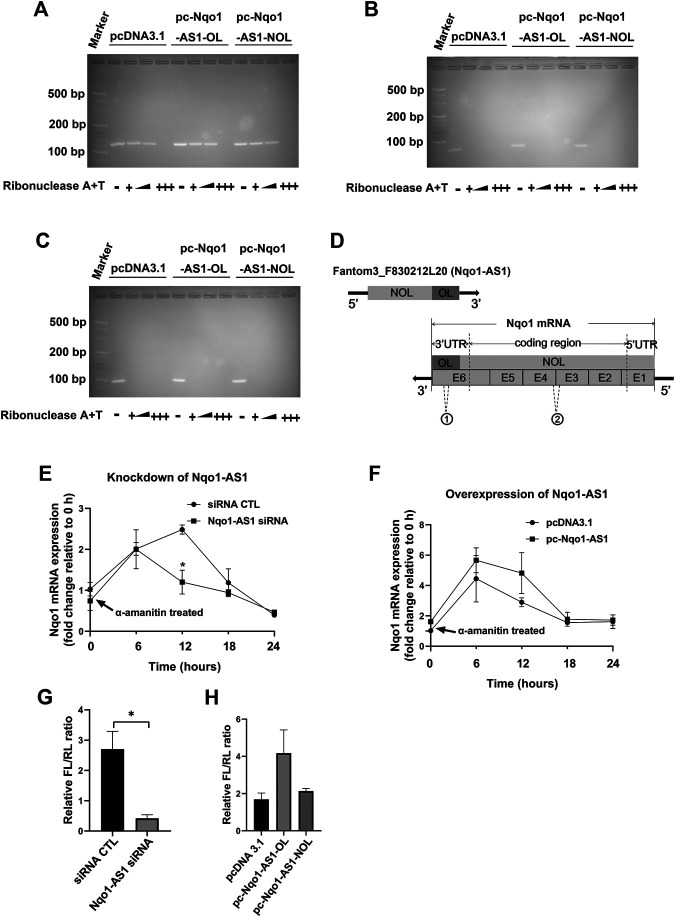
FIGURE 8.
Nqo1-AS1 increases Nqo1 mRNA stability and expression. (A–C) RNase protection assay was performed to examine the RNA duplexes formation between Nqo1-AS1 and Nqo1 mRNA. PcDNA3.1-Nqo1-AS1 overlapping region (pc-Nqo1-AS1-OL), PcDNA3.1-Nqo1-AS1 non-overlapping region (pc-Nqo1-AS1-NOL) or pcDNA3.1 (control) vector was cotransfected with pcDNA3.1- Nqo1 (pc-Nqo1) into mle-12 cells. After transfection for 48 h, the total RNA was extracted from the cells. RNA sample was digested with increasing amounts of RNAse A + T cocktail (represented as the black wedge and multiple “+++”) in various samples.Then the remaining double-stranded RNA was reversely transcribed to cDNA and amplified the overlapping part of Nqo1 (Nqo1-OL) mRNA (A) and the non-overlapping part of Nqo1 (Nqo1-NOL) mRNA (B) by PCR. Gapdh PCR product (C) was used as a control. (D) Schematic diagram displayed the RNA duplexes formation between the overlapping part of Nqo1-AS1 (Nqo1-AS1-OL) and the overlapping part of Nqo1 (Nqo1-OL) mRNA, which protected both of them from RNase digestion. “E” followed by number represented exon. The sites of primers used in RNase protection assay were indicated as follows:1 Nqo1-OL PCR primer; 2Nqo1-NOL PCR primer. (E–F) Line chart shown the stability of Nqo1 mRNA over time relative to time 0 after blocking new RNA synthesis with a-amanitin treatment (10 μg/ml). 18S rRNA was used as an internal control, which was a product of RNA polymerase I and was unchanged after a-amanitin treatment. (E) Nqo1-AS1 siRNA or siRNA CTL was transfected into the mle-12 cells for 24 h, and followed by a-amanitin treatment for 0 h, 6 h, 12 h, 18 and 24 h. Then the Nqo1 mRNA expression level was measured by qRT-PCR. (F) The pc-Nqo1-AS1 or pcDNA3.1 vector was transfected into the mle-12 cells for 24 h and then treated with a-amanitin for 0 h, 6 h, 12 h, 18 and 24 h. Subsequently, the Nqo1 mRNA expression level was detected by qRT-PCR. (G) The luciferase activity of pmirGLO-Nqo1 3′UTR was markedly decreased in the mle-12 cells transfected with Nqo1-AS1 siRNA compared to cells transfected with siRNA CTL. (H) The luciferase activity of pmirGLO-Nqo1 3′UTR was increased significantly in the mle-12 cells that overexpressing the Nqo1-OL of Nqo1-AS1, but not the Nqo1-NOL of Nqo1-AS1. *p < 0.05. Data represented the mean ± SEM from three independent experiments.