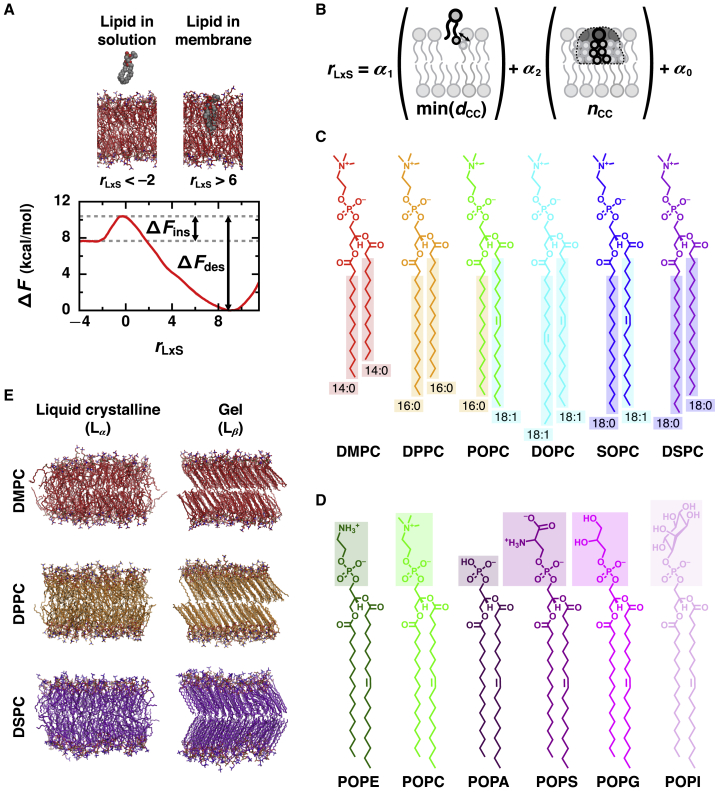
Figure 1.
Physicochemical properties that determine the rate of lipid transport were assessed for a variety of lipid chemistries and phases by calculating the free energy barriers for lipid desorption and insertion. (A) An illustrative free energy profile, calculated as a function of the reaction coordinate, rLxS, for an Lα phase DMPC membrane, reveals barriers for lipid desorption (ΔFdes) and lipid insertion (ΔFins). Representative configurations are shown for negative values of rLxS (at which the lipid is in solution) and for positive values of rLxS (at which the lipid is in the membrane). Solvent is not rendered. (B) rLxS is a linear combination of min(dCC) (the minimal distance between any hydrophobic carbon of the lipid and of the closest membrane leaflet) and nCC (the number of close hydrophobic carbon contacts between the lipid and closest leaflet). (C and D) Chemical structures of the lipids investigated with varied (C) acyl chain lengths and degrees of saturation and (D) headgroups. POPC’s structure is shown in both (C) and (D). In (C), hydrophobic carbons are boxed. (E) Representative configurations of membranes composed of DMPC, DPPC, or DSPC in both Lα and Lβ phases. To see this figure in color, go online.