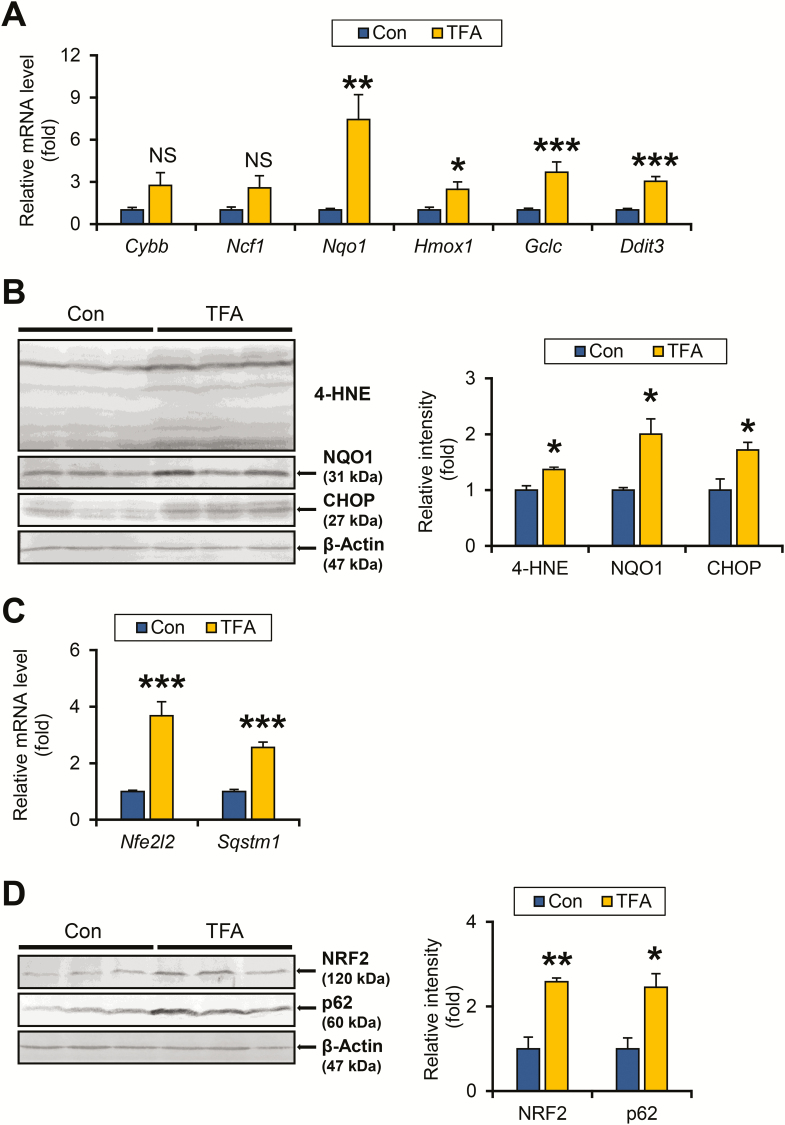
Figure 3.
TFA-rich diet enhances oxidative/ER stress and activated NRF2-p62 signaling in HCVcpTg mice. (A) Hepatic mRNA levels of genes related to oxidative stress (Cybb, Ncf1, Nqo1, Hmox1 and Gclc) and ER stress (Ddit3) were quantified by qPCR, normalized to those of 18S ribosomal RNA and expressed as values relative to HCVcpTg mice fed a control diet. (B) Immunoblot analysis of 4-hydroxy-nonenal, NQO1 and C/EBP-homologous protein. Whole-liver homogenates (60–80 μg of protein) were loaded into each well and the band of β-actin was used as a loading control. Band intensities were measured densitometrically, normalized to those of β-actin and expressed as values relative to control diet-fed HCVcpTg mice. Results were obtained from two independent immunoblot experiments and representative blots were shown. (C) Hepatic mRNA levels of genes encoding NRF2 (Nfe2l2) and p62 (Sqstm1) were quantified by qPCR, normalized to those of 18S ribosomal RNA and expressed as values relative to HCVcpTg mice fed a control diet. (D) Immunoblot analysis of NRF2 and p62. Whole-liver homogenates (60–80 μg of protein) were loaded into each well and the band of β-actin was used as a loading control. Band intensities were measured densitometrically, normalized to those of β-actin and expressed as values relative to HCVcpTg mice treated with a control diet. Results were obtained from two independent immunoblot experiments and representative blots were shown. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 between control diet-fed and TFA-rich diet-fed HCVcpTg mice. Con, control diet-fed HCVcpTg mice; TFA, TFA-rich diet-fed HCVcpTg mice; NS, not significant.