Abstract
Evolutionary adaptation is a major source of antibiotic resistance in bacterial pathogens. Evolution-informed therapy aims to constrain resistance by accounting for bacterial evolvability. Sequential treatments with antibiotics that target different bacterial processes were previously shown to limit adaptation through genetic resistance trade-offs and negative hysteresis. Treatment with homogeneous sets of antibiotics is generally viewed to be disadvantageous as it should rapidly lead to cross-resistance. We here challenged this assumption by determining the evolutionary response of Pseudomonas aeruginosa to experimental sequential treatments involving both heterogenous and homogeneous antibiotic sets. To our surprise, we found that fast switching between only β-lactam antibiotics resulted in increased extinction of bacterial populations. We demonstrate that extinction is favored by low rates of spontaneous resistance emergence and low levels of spontaneous cross-resistance among the antibiotics in sequence. The uncovered principles may help to guide the optimized use of available antibiotics in highly potent, evolution-informed treatment designs.
Research organism: Other
eLife digest
Overuse of antibiotic drugs is leading to the appearance of antibiotic-resistant bacteria; this is, bacteria with mutations that allow them to survive treatment with specific antibiotics. This has made some bacterial infections difficult or impossible to treat. Learning more about how bacteria evolve resistance to antibiotics could help scientists find ways to prevent it and develop more effective treatments.
Changing antibiotics frequently may be one way to prevent bacteria from evolving resistance. That way if a bacterium acquires mutations that allow it to escape one antibiotic, another antibiotic will kill it, stopping it from dividing and preventing the appearance of descendants with resistance to several antibiotics. In order to use this approach, testing is needed to find the best sequences of antibiotics to apply and the optimal timings of treatment.
To find out more, Batra, Roemhild et al. grew bacteria in the laboratory and exposed them to different sequences of antibiotics, switching antibiotics at different time intervals. This showed that sequential treatments with different antibiotics can limit bacterial evolution, especially when antibiotics are switched quickly. Unexpectedly, one of the most effective sequences used very similar antibiotics. This was surprising because using similar antibiotics should lead to the evolution of cross-resistance, which is when a drug causes changes that make the bacterium less sensitive to other treatments. However, in the tested case, cross-resistance did not evolve when antibiotics were switched quickly, thereby ensuring efficiency of treatment.
Batra et al. show that alternating sequences of antibiotics may be an effective strategy to prevent drug resistance. Because the experiments were done in a laboratory setting it will be important to verify the results in studies in animals and humans before the approach can be used in medical or veterinary settings. If the results are confirmed, it could reduce the need to develop new antibiotics, which is expensive and time consuming.
Introduction
The efficacy of antibiotics for the treatment of infections is diminishing rapidly as bacteria evolve new mechanisms to resist antibiotics (Laxminarayan et al., 2013). Resistance evolution is frequently observed during antibiotic therapy and can happen within days (Bloemberg et al., 2015; Hjort et al., 2020; Tueffers et al., 2019). A failure to account for such rapid bacterial adaptation is likely a common reason for treatment failure (Woods and Read, 2015; Zhou et al., 2020). For this reason, the field of evolutionary medicine specifically accounts for bacterial evolvability and seeks treatment solutions that are hard to overcome by genetic adaptation (Andersson et al., 2020; Merker et al., 2020). While an evolution-proof antibiotic remains to be found, the mechanisms that restrict evolutionary escape are starting to be revealed (Bell and MacLean, 2018). Such evolutionary insight may guide the design of effective and sustainable antibiotic therapy.
An effective way of reducing the amount of evolutionary solutions is to administer several antibiotics either simultaneously (i.e., combination therapy) or sequentially (i.e., sequential therapy). Tailored combination treatments make use of physiological and evolutionary constraints (Baym et al., 2016). The emergence of resistance is delayed by combinations, when evolutionary escape requires multiple mutations and when drug interactions eliminate the intermediate genetic steps of single-drug resistance (Chait et al., 2007), antibiotic tolerance (Levin-Reisman et al., 2017), and heteroresistance (Band et al., 2019). However, when genetic resistance to the combination is easily accessible, for example, through gene amplification of efflux pumps, then combination therapy can accelerate resistance emergence (Pena-Miller et al., 2013). This undesired selective effect is potentially avoided by sequential drug application. Evolutionary escape from sequential treatments is constrained by negative hysteresis responses induced by specific antibiotics (Roemhild et al., 2018) and/or the emergence of genetic collateral sensitivity trade-offs (Barbosa et al., 2019; Yoshida et al., 2017). Negative hysteresis occurs when exposure to an antibiotic induces changes to bacterial physiology that transiently increase the killing efficacy of other antibiotics (Roemhild et al., 2018). Collateral sensitivity is a genetic side effect of evolved resistance that too increases the efficacy of other antibiotics (Szybalski and Bryson, 1952). Collateral sensitivity is prevalent among pathogens and occurs especially between antibiotics with distinct mechanism of action (i.e., heterogeneous sets of antibiotics), while cross-resistance often emerges towards antibiotics with similar mode of action (i.e., homogeneous sets of antibiotics) (Barbosa et al., 2017; Imamovic and Sommer, 2013; Lázár et al., 2013; Maltas and Wood, 2019). Thus, conventionally, multidrug treatments would avoid antibiotics from similar classes, with the rationale of limiting the overlap in the respective sets of resistance mutations, and thus the ensuing cross-resistance.
The particular efficacy of sequential therapy has been confirmed with the help of evolution experiments under controlled laboratory conditions. Different types of sequential treatments have been tested. Some regimens involved a single switch between antibiotics, while others included multiple switches at short time intervals. One of the main findings was that the efficacy of sequential treatments depended both on the included antibiotics and the particular treatment sequence (Fuentes-Hernandez et al., 2015; Maltas and Wood, 2019; Roemhild et al., 2015). While fast sequential treatments did not exclude the eventual emergence of multidrug resistance, many significantly delayed bacterial adaptation compared to monotherapy (Kim et al., 2014; Roemhild et al., 2015; Yoshida et al., 2017). A single antibiotic switch can also delay adaptation, dependent on the drug order, and it can additionally reverse previous resistance and resensitize bacterial populations to specific antibiotics (Barbosa et al., 2019; Hernando-Amado et al., 2020; Imamovic and Sommer, 2013; Yen and Papin, 2017). Moreover, our group previously demonstrated that fast sequential treatments with a heterogeneous set of three antibiotics – the fluoroquinolone ciprofloxacin (CIP), the β-lactam carbenicillin (CAR), and the aminoglycoside gentamicin (GEN) – delayed the emergence of multidrug resistance in the pathogen Pseudomonas aeruginosa (Roemhild et al., 2018). The observed inhibition of evolutionary escape was manifested by the occurrence of population extinction, although antibiotic concentrations were below the minimal inhibitory concentration (MIC). We further found that negative hysteresis at antibiotic switches reduced adaptation rates because it selected for distinct genetic changes. Several populations adapted to fast sequential treatment by independent mutations in the histidine kinase cpxS that only mildly increased resistance, thereby explaining the low rate of adaptation to the used antibiotics. Instead, the cpxS mutations suppressed negative hysteresis, demonstrating that adaptation was specific to the selective constraint imposed by the drug switches. Based on these findings, we assumed that the acting selective dynamics were ultimately a consequence of antibiotic heterogeneity. However, is this so? Do selective dynamics differ for a homogenous set of drugs?
The primary aim of our current study was to assess the efficacy of sequential treatments with either heterogeneous or homogeneous sets of three antibiotics. We focused on P. aeruginosa strain PA14 as a tractable pathogen model system, for which comprehensive experimental reference data is available on resistance evolution (e.g., Barbosa et al., 2019; Barbosa et al., 2018; Hernando-Amado et al., 2020; Roemhild et al., 2018; Sanz-García et al., 2018; Yen and Papin, 2017). We performed similar evolution experiments as before, with three new sets of bactericidal antibiotics, two of which included only β-lactams, and one the three previously considered modes of action (Figure 1—figure supplement 1A). The new heterogeneous drug set CIP, streptomycin (STR), and doripenem (DOR) involved drug synergy and was expected to contribute to collateral sensitivity (Barbosa et al., 2018; Barbosa et al., 2017). The drug sets comprising three β-lactams, however, had all properties that would typically be avoided for the design of multidrug treatments. The three β-lactams CAR, cefsulodin (CEF), and DOR have the same core structure and individually inhibit the DD-transpeptidase activity in cell-wall synthesis (Walsh, 2003). The collateral effects landscape between CAR-CEF-DOR was expected to be dominated by cross-resistance (Barbosa et al., 2017) and the three antibiotics showed neither synergy nor antagonism (Barbosa et al., 2018). Resistance to these antibiotics may potentially be achieved through single mutations. The situation is replicated by the set of ticarcillin (TIC), azlocillin (AZL), and ceftazidime (CEZ). In contrast to expectations, the triple β-lactam sequences showed high treatment potency. Therefore, the secondary aim of our study was to assess which characteristics constrained the ability of the bacteria to adapt to the β-lactam sequential treatments. We focused on one triple β-lactam set (CAR-CEF-DOR) and specifically tested the influence of antibiotic switching rate, switching regularity, negative hysteresis, the potential for spontaneous resistance evolution, and resulting cross-resistances on treatment efficacy.
Results
Triple β-lactam sequential treatments favor extinction of bacterial populations
We challenged a total of 756 replicate P. aeruginosa populations with sequential treatments across three fully independent evolution experiments, each focused on a different set of three antibiotics (Figure 1, Figure 1—figure supplement 1, Supplementary file 1A, Materials and methods). The antibiotic concentrations were calibrated to an inhibitory concentration of 75% (IC75), allowing bacteria to adapt to the imposed selection pressure. We used a serial dilution protocol for experimental evolution, with 2% culture transfer after 12 hr (one transfer) across a total of 96 transfers, equivalent to approximately 500 bacterial generations. Following the previous setup (Roemhild et al., 2018), we recorded the evolutionary dynamics in response to 16 different treatments, belonging to four main treatment types: monotherapy, fast-regular, slow-regular, and random sequential therapy (Figure 1).
Figure 1. Probability of evolutionary rescue depends on drug triplets and treatment type.
(A) The evaluated antibiotic combinations comprise different types of antibiotic targets. Fluoroquinolone antibiotics (FQ) target DNA gyrase, aminoglycosides (AG) inhibit translation, and β-lactams (BL) inhibit cell-wall synthesis. (B) The evaluated treatment protocols test the effects of switching rate and temporal regularity. (C) A fraction of lineages is eradicated by the sublethal dosage sequential treatments. Lineage extinction is high for combinations of cell-wall targeting β-lactams. (D) Variation in extinction for the β-lactam combinations by treatment type (n = 3–6 protocols per treatment type). (E) The distribution of evolutionary trajectories for Exp. 3 with CAR-DOR-CEF shows that the majority of extinction events occur within the first 12 serial transfers (n = 180 lineages). Growth of evolving lineages is quantified relative to untreated reference populations using the relative area under the growth curve (AUC). AZL: azlocillin; CAR: carbenicillin; CEF: cefsulodin; CEZ: ceftazidime; CIP: ciprofloxacin; DOR: doripenem; GEN: gentamicin; STR: streptomycin; TIC: ticarcillin. The following supplementary material is available for Figure 1: Figure 1—figure supplement 1, Figure 1—source data 1, Figure 1—figure supplement 1—source data 1, Supplementary file 1A.
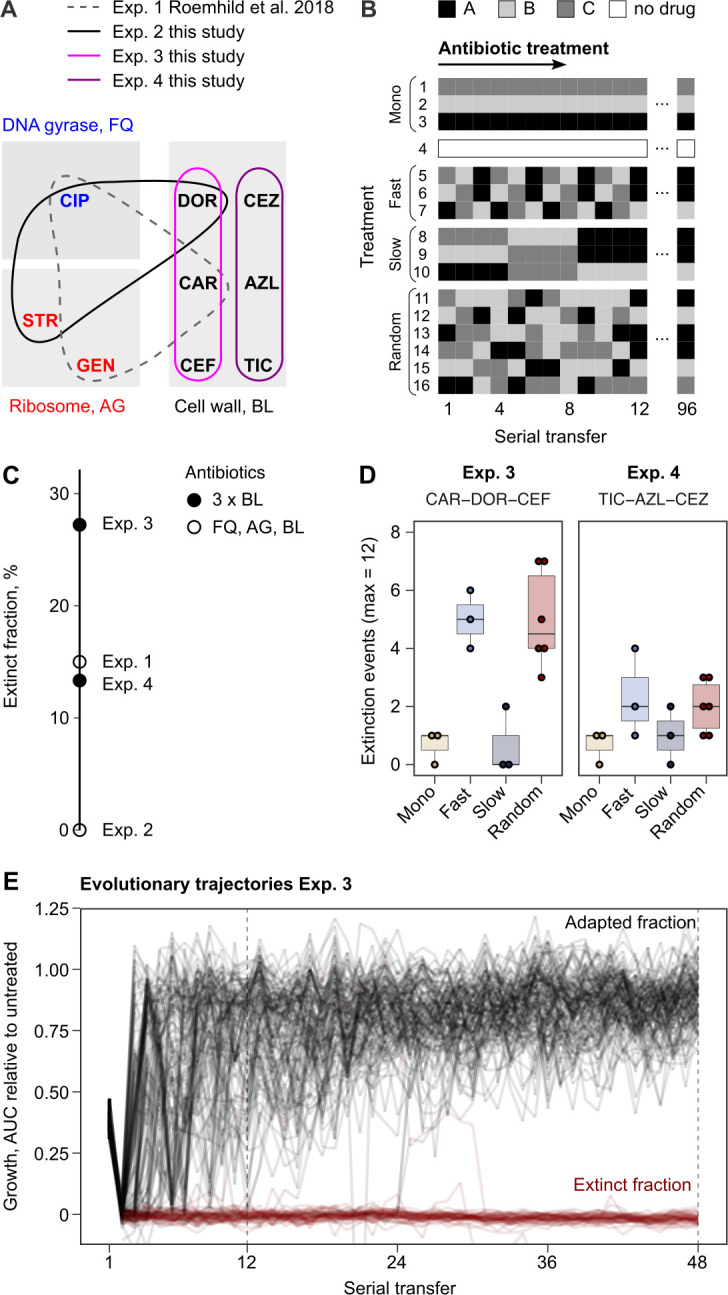
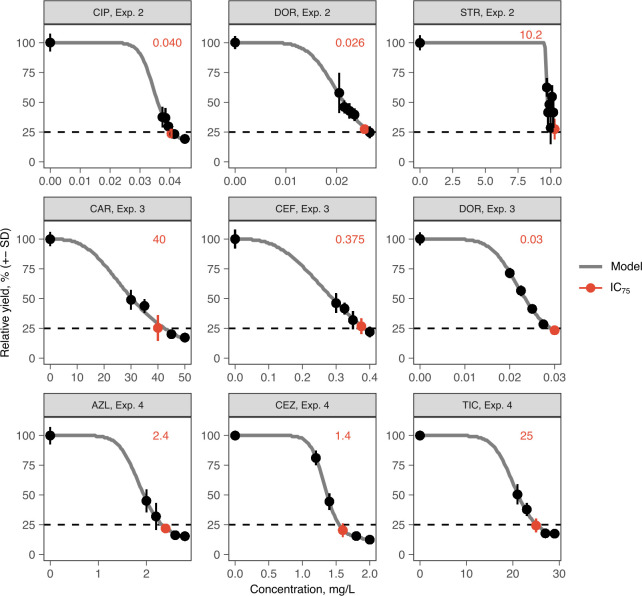
Figure 1—figure supplement 1. Antibiotic dose-response curves for PA14 (mean ± s.d.; n = 6 biological replicates).
Extinction of experimental populations differed considerably between the antibiotic sets. The two β-lactam sets produced a surprisingly high degree of extinction (CAR-CEF-DOR and TIC-AZL-CEZ; extinct fraction 27.2 and 13.3%, respectively, Figure 1C). The observed extinction frequency was comparable to that observed in the previous experiment with CAR-CIP-GEN (extinct fraction 15%, Figure 1C). CIP-DOR-STR caused no extinction, indicating that extinction was not explained by applying heterogeneous sets of antibiotics. Within the β-lactam sequential treatments, we observed that treatments that switched between antibiotics fast (every transfer) produced much higher extinction levels than those that switched slowly (every four transfers) or not at all (Figure 1D). Most of the extinction events happened early in the experiment (Figure 1E), indicating that the initial treatment steps are critical for adaptation of populations. We conclude that fast sequential β-lactam treatments showed a surprising ability to restrict bacterial adaptation. As this result was unexpected, we decided to research the mechanisms that constrain resistance emergence in β-lactam sequences. Given that the experiment involving CAR-CEF-DOR produced the highest fraction of extinct populations, we decided to focus further analyses on this set.
Resistance to doripenem was constrained in both monotherapy and switching treatments in the CAR-CEF-DOR triple β-lactam experiment
The CAR-CEF-DOR triple β-lactam experiment was characterized in detail for changes in growth, evolved resistance, and whole-genome sequences in order to assess the selection dynamics involved. We calculated the relative growth yield (see Materials and methods) at the end of each transfer and found growth dynamics to be divided into three phases: an early phase of rapid adaptation (transfers 1–12), followed by a phase of gradual growth yield convergence (transfers 13–48), and a final plateau phase (transfers 49–96) (Figure 2A; the growth phases are separated by vertical dotted lines). We compared the main treatment types using general linear models (GLM) for each phase separately (this fulfills the model assumption of response linearity). The early phase dynamics were characterized by significantly decelerated adaptation dynamics of the fast-regular group compared with monotherapy and slow-regular (GLM, post hoc test, p<0.037, Supplementary file 1B), but not random treatments. The slow-regular treatment did not differ significantly from monotherapy or random treatments (GLM, post hoc test, p=0.469, Supplementary file 1B). In the subsequent phase, growth yields of the groups converged to a plateau of roughly 90% relative yield, indicating similar final levels of adaptation (the growth yields of main treatment groups showed no statistical differences in phases 2 and 3, Supplementary file 1B). Alternating between the β-lactams fast and in a regular order therefore significantly constrained the growth of the bacterial populations. Intriguingly, in these fast sequential treatments, bacterial growth in the transfers with DOR was lower than in the transfers with the other two antibiotics (Figure 2—figure supplement 1), indicating an evolutionary constraint associated with the antibiotic DOR. We can rule out the alternative hypotheses that the reduced growth is explained by a stronger initial reduction in bacterial population size by DOR in comparison to the other two drugs or increased stochastic variation in dosage effects. All treatments were initiated using specifically standardized IC75 dosage (see Materials and methods) and at the IC75, DOR showed very little variation (Figure 1—figure supplement 1). We thus hypothesize that the observed evolutionary constraint may be due to lower rate of DOR resistance emergence.
Figure 2. Resistance to doripenem is constrained in the CAR-CEF-DOR triple β-lactam experiment.
(A) Rapid adaptive increase of biomass yields relative to the untreated reference populations (mean ± CI95; n = 3–6 protocols per treatment type and 12 biological replicates per sequence; extinct lineages excluded). Vertical dotted lines separate the three growth phases. Evolved changes in the susceptibility to the treatment antibiotics CAR, DOR, and CEF and the non-treatment antibiotics CIP and GEN after transfer 12 (B) or transfer 48 (C), evaluated with 20 isolates each for the 16 representative adapting populations at each time point. Mono 1 is monotherapy with CAR, mono 2 is monotherapy with DOR, and mono 3 is monotherapy with CEF. The evolution of resistance and hypersensitivity is indicated by red and blue colors, respectively, given for the considered isolates as horizontal lines (total of 640 isolates), sorted according to evolution treatment (main rows in the figures) and tested antibiotics (main columns; antibiotics given at the bottom). Pie charts on the right show phenotypic within-population diversity, where different colors indicate subpopulations inferred from hierarchical clustering of resistance phenotypes. CAR: carbenicillin; CEF: cefsulodin; CIP: ciprofloxacin; DOR: doripenem; GEN: gentamicin. The following supplementary material is available for Figure 2: Figure 2—figure supplement 1, Figure 2—figure supplement 2, Figure 2—figure supplement 3, Figure 2—figure supplement 4, Figure 2—figure supplement 5, Figure 2—source data 1, Figure 2—figure supplement 1—source data 1, Figure 2—figure supplement 2—source data 1, Figure 2—figure supplement 3—source data 1, Figure 2—figure supplement 5—source data 1, Supplementary file 1B.
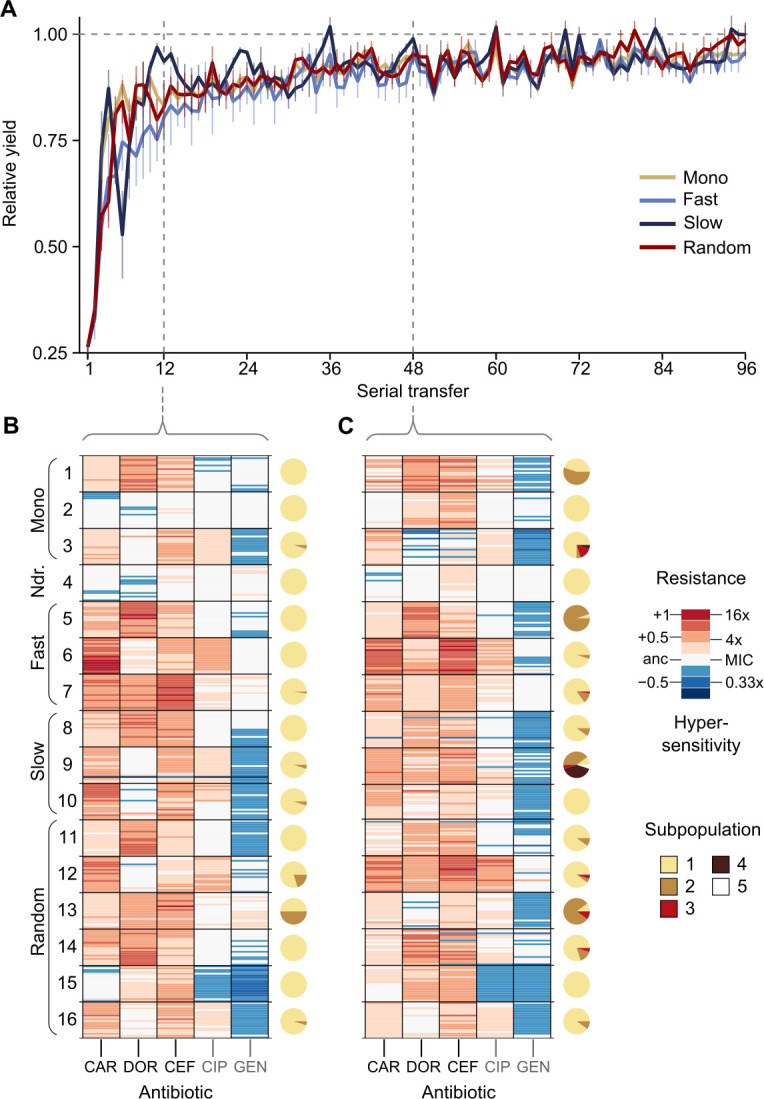
Figure 2—figure supplement 1. Growth dynamics in fast sequential protocols.
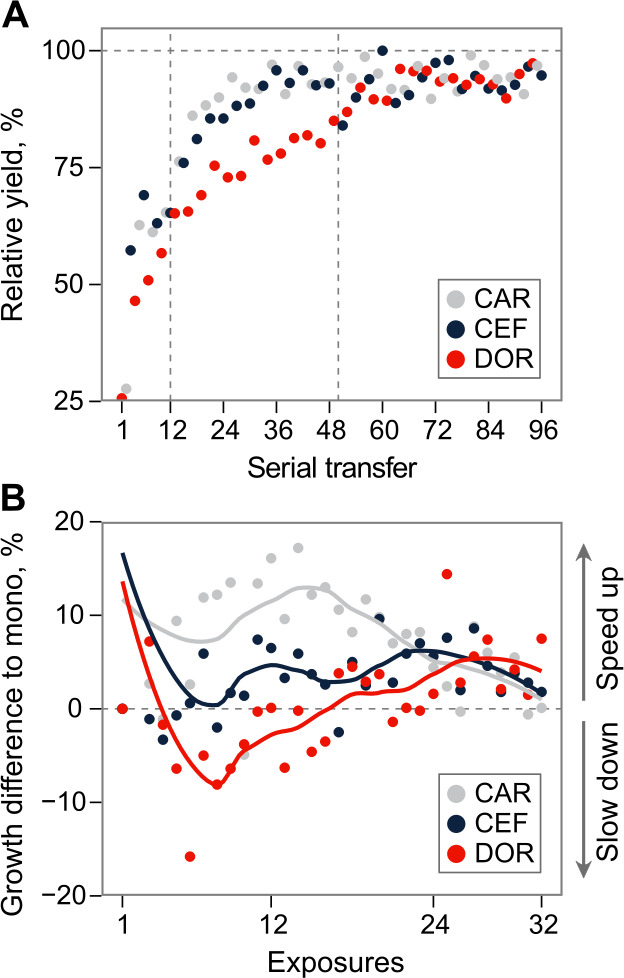
Figure 2—figure supplement 2. Dose-response curve distributions for Exp. 3 with CAR-DOR-CEF, underlying Figure 2B and C.
Figure 2—figure supplement 3. Relation between the resistance values and the fold-change of the minimal inhibitory concentrations (MIC) approximated by IC90.
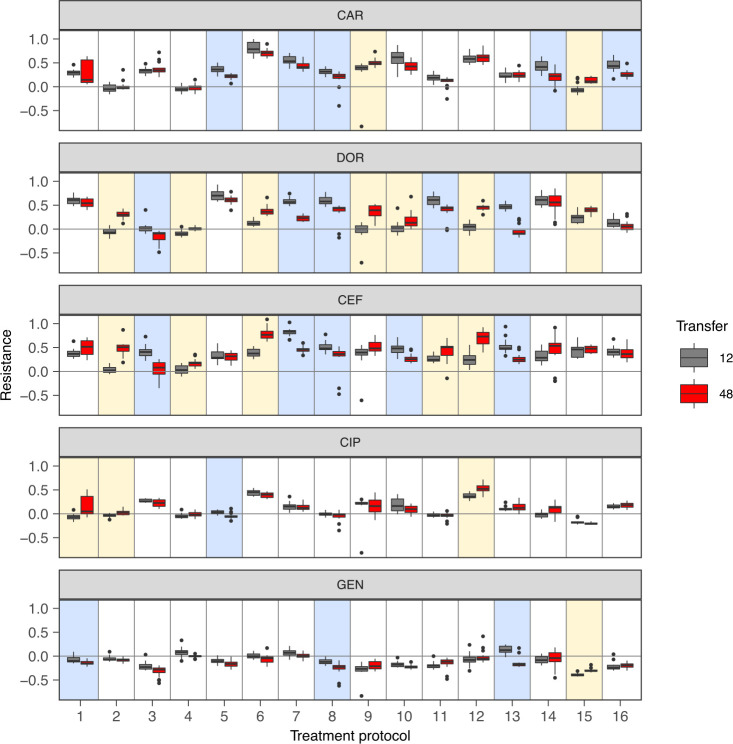
Figure 2—figure supplement 4. Change of population antibiotic resistance between transfer 12 (indicated in gray) and transfer 48 (indicated in red).
Figure 2—figure supplement 5. Population multidrug resistance after (A) transfer 12 and (B) transfer 48.
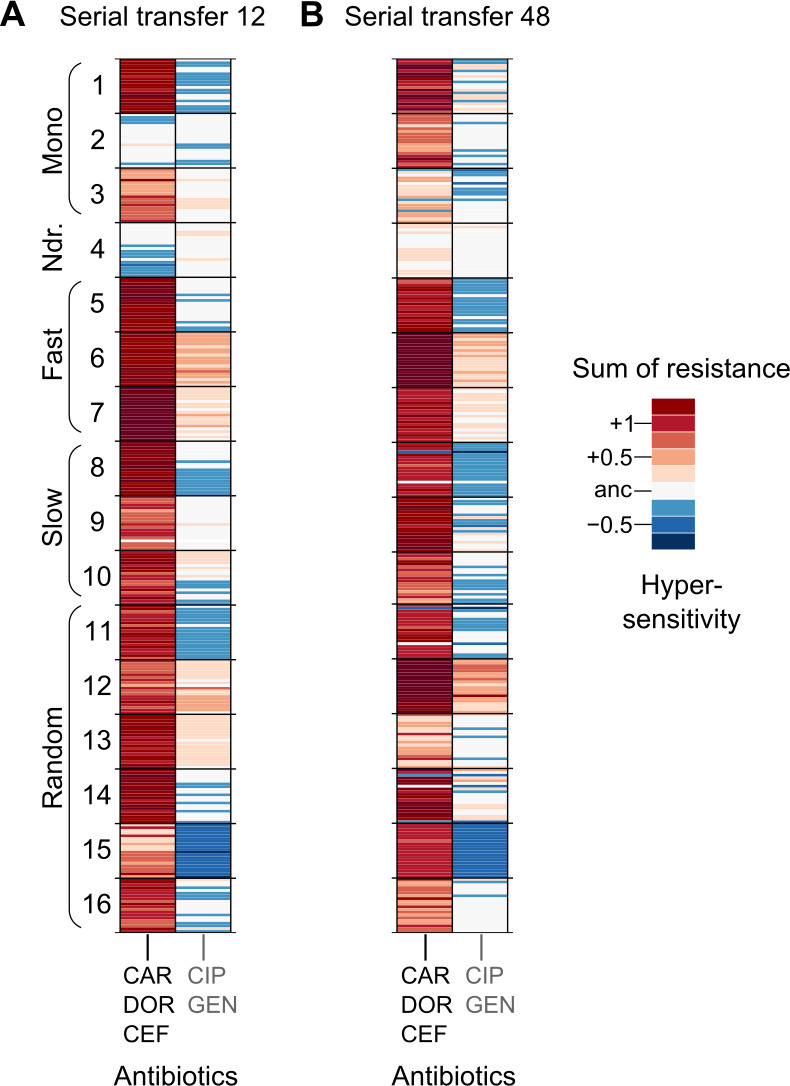
To understand the dynamics of early adaptation in more detail, we measured the resistance profiles of 16 evolved populations after transfers 12 and 48 from the different antibiotic treatments (representing the end of phases 1 and 2, respectively; Figure 2B, C, Figure 2—figure supplements 2–5, Supplementary file 1B–F; see Materials and methods). We randomly sampled 20 bacterial colonies from each population and characterized their resistance profile by broth microdilution. Resistance was measured for the three antibiotics of the evolution experiment and two additional clinically relevant antibiotics from different classes, ciprofloxacin and gentamicin. The resistance profiles in the early and the mid phases were found to be distinctly different. Resistance to the used β-lactams increased across the two time points only in some treatments, but not all (Figure 2B, C, Figure 2—figure supplement 4, Figure 2—figure supplement 5, Supplementary file 1F), suggesting treatment-dependent evolutionary responses to the antibiotics. We assessed how the main treatment types varied in their β-lactam resistance using a GLM for each phase separately. Most treatment types varied significantly from each other in their multidrug β-lactam resistance in both phases (Supplementary file 1C, D). The multidrug resistance in the early phase was in most cases constrained by the susceptibility to DOR (e.g., in the switching and monotherapy treatments). We additionally observed collateral responses of the treatment to the two non-β-lactams, which increased over time. We further used hierarchical clustering of the resistance profiles to assess the presence of subpopulations, followed by calculation of Shannon diversity for each population at both transfers. We found population diversity to be significantly higher at transfer 48 as compared to transfer 12 (ANOVA, F = 6.2060, p=0.01893, Supplementary file 1E), indicating a diversification of the evolving lineages over time. Taken together, the population analysis of resistance profiles indicates that resistance evolution depends on the exact treatment protocol and that the dynamics of resistance emergence to DOR may be key for the observed deceleration of β-lactam adaptation in the fast-regular treatments.
To identify the genomic changes underlying the first steps of β-lactam adaptation, we sequenced 33 whole genomes of the evolved and characterized isolates from the monotherapy, fast-regular, and slow-regular treatment types. Specifically, we sequenced three isolates from each population representing the distinct phenotypic subpopulations, assessed above. We found that all isolates, except those that received DOR monotherapy, had mutations in known resistance genes by the end of the early phase (Table 1). This agreed with the inferred resistance profiles where isolates from the DOR monotherapy did not show a noticeable amount of resistance at that stage (Figure 2B). DOR resistance was, however, found at the end of the middle phase (Figure 2C), and this was mirrored in the genomics with a non-synonymous mutation in the gene ftsI. This gene codes for the penicillin binding protein 3 (PBP3) (Liao and Hancock, 1995), a common target of the three β-lactams (Davies et al., 2008; Fontana et al., 2000; Rodriguez‐Tebár et al., 1982; Rodríguez-Tebar et al., 1982; Zimmermann, 1980). ftsI was also found to be mutated in isolates from CAR monotherapy, although at a different site within the gene and associated with a different resistance profile than the DOR-associated ftsI variant (Figure 2B). Isolates from CEF monotherapy contained mutations in pepA. This gene is responsible for the production of a protein required for cytotoxicity and virulence in P. aeruginosa (Hauser et al., 1998). Although its role in antimicrobial resistance remains to be studied in detail, it was previously found to be mutated in P. aeruginosa strains resistant to certain β-lactams (Cabot et al., 2018; Sanz-García et al., 2018). The switching treatments selected for mutations in the above-listed and also in some additional genes. In particular, we identified mutations in nalD and phoQ, a negative regulator of the MexAB-OprM efflux pump and a two-component system, respectively. Mutations in these genes account for resistance to a variety of drugs in P. aeruginosa (Barbosa et al., 2021; Sobel et al., 2005). Further mutations were identified in some non-canonical β-lactam resistance genes such as rmcA, 23srRNA, 3-oxoacyl synthase, dnaX, and zipA (Table 1). Taken together, mutations in both canonical and non-canonical targets of β-lactam selection were identified in our experiment, and among these, DOR resistance mutations were found only later in the experiment, consistent with the obtained resistance profiles (Figure 2B, C).
Table 1. Evolved genetic changes inferred from whole-genome sequencing.
| Treatment type | ID* | AA change† | Gene name | Annotation | Freq‡ |
|---|---|---|---|---|---|
| Monotherapy | 1 | V471G | ftsI | Peptidoglycan synthesis | 3/3 |
| 2§ | N242S | ftsI | Peptidoglycan synthesis | 3/3 | |
| 3 | T157P | pepA | Virulence | 3/3 | |
| Fast-rgular | 5 | V471G | ftsI | Peptidoglycan synthesis | 3/3 |
| 6 | K26 | nalD | Efflux | 3/3 | |
| S379ISR | rmcA | Biofilm maintenance | 1/3 | ||
| 7 | R220C | phoQ | Two-component | 3/3 | |
| - | PA14_55631 | 23srRNA, translation | 1/3 | ||
| Slow-rgular | 8 | V471G | ftsI | Peptidoglycan synthesis | 3/3 |
| 9 | D357N | pepA | Virulence | 3/3 | |
| 10 | T157P | pepA | Virulence | 3/3 | |
| E115VAAWIPK | PA14_21540 | Lipid metabolism (3-exoacyl ACP synthase) | 1/3 | ||
| Q117AEEQ | PA14_21540 | Lipid metabolism (3-exoacyl ACP synthase) | 1/3 | ||
| R178C | zipA | Cell division | 2/3 | ||
| P483PEP | dnaX | Cell division | 1/3 |
* Individual treatment of evolution experiment.
† Amino acid change.
‡ Occurrence frequency of the identified variant (before slash) out of the total number of isolates sequenced (behind slash).
§ Mutations listed are from isolates obtained from the populations frozen at transfer 48, no variants were found in the isolates from transfer 12.
Based on our detailed characterization of the CAR-CEF-DOR triple β-lactam experiment, we conclude that DOR has a key role in restricting evolutionary rescue as evidenced by the delayed acquisition of genetic resistance to it.
Asymmetric bidirectional hysteresis was identified between doripenem and carbenicillin
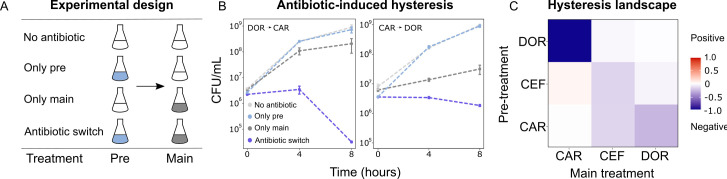
As extinction was associated with antibiotic switches, we next focused on selective events that can occur at drug switches, such as hysteresis, an inducible physiological change. We characterized the complete hysteresis landscape between the three β-lactams: CAR, DOR, and CEF. We pretreated exponential phase cells with an antibiotic for only 15 min to ensure that cells are physiologically challenged but not subject to differential killing or replication. The pretreatment was followed by a change to fresh medium containing a second antibiotic as main treatment. We included controls of no pretreatment, or no main treatment (Figure 3A). We found that negative hysteresis existed for several switches between the β-lactams (Figure 3B, C, Figure 3—figure supplement 1, Figure 3—figure supplement 2). DOR and CAR displayed asymmetric bidirectional negative hysteresis with the switch from DOR to CAR, resulting in stronger negative hysteresis than the reverse. Negative hysteresis was also observed in the switch from CAR to CEF and CEF to CEF. To our surprise, only a single case of weak positive hysteresis was observed, although we generally anticipated it given that P. aeruginosa produces the AmpC β-lactamase (Livermore, 1995). We conclude that negative hysteresis is abundant between the studied β-lactams and is a potential predictor of treatment potency in the sequential β-lactam treatments.
Figure 3. Negative hysteresis is common among the tested β-lactam antibiotics.
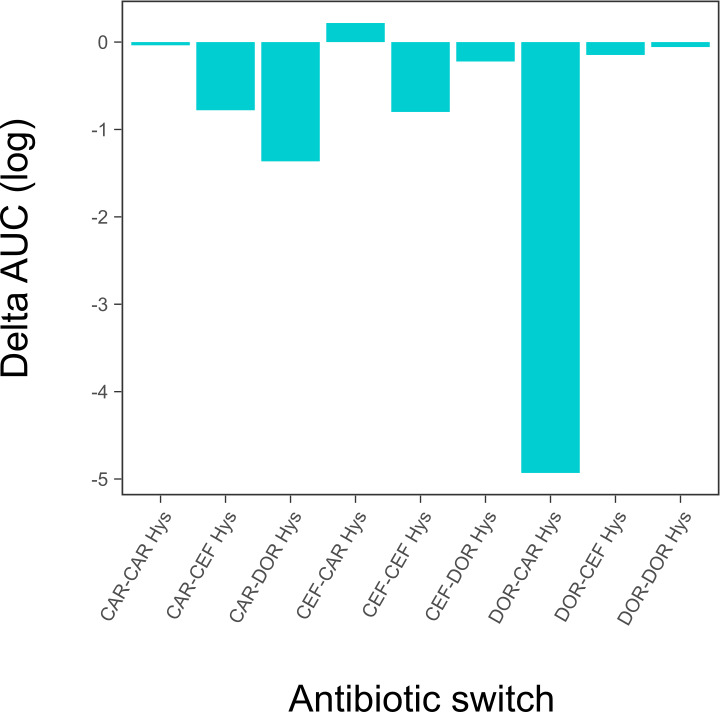
(A) Hysteresis effects were measured using the previously established experimental approach (see Materials and methods). (B) Bacterial counts were plotted over time after the pretreatment to obtain time-kill curves (mean ± sem, n = 3). Level of hysteresis was quantified as the difference between the antibiotic switch and the only main curves. Negative values indicate negative hysteresis and positive values indicate positive hysteresis. (C) Heatmap of hysteresis levels between all nine combinations of the three β-lactams. DOR and CAR show asymmetric bidirectional negative hysteresis. Negative hysteresis is also observed in switches from CEF to CEF and CAR to CEF. Weak positive hysteresis is found for the switch from CEF to CAR. The following supplementary material is available for Figure 3: Figure 3—figure supplement 1, Figure 3—figure supplement 2, Figure 3—source data 1, Figure 3—figure supplement 1—source data 1. CAR: carbenicillin; CEF: cefsulodin; DOR: doripenem.
Figure 3—figure supplement 1. Time-kill curves of hysteresis experiments for the combinations not presented in Figure 3 (mean ± sem, n = 3).
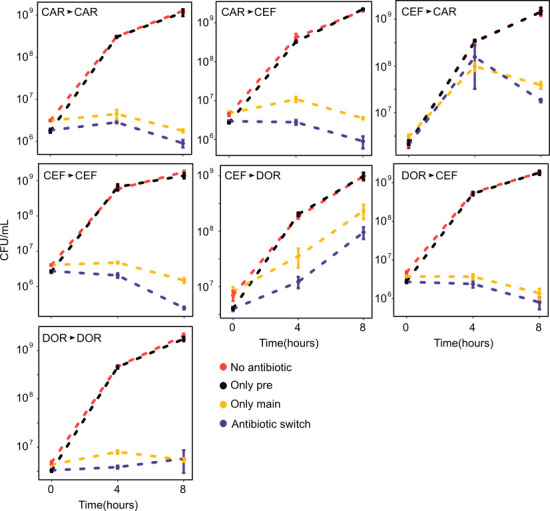
Figure 3—figure supplement 2. Hysteresis effects quantified as area under the curve (AUC) difference between the ‘only main’ and ‘antibiotic switch’ curves from the time-kill dynamics.
Probability of direct and indirect resistance was the least for doripenem
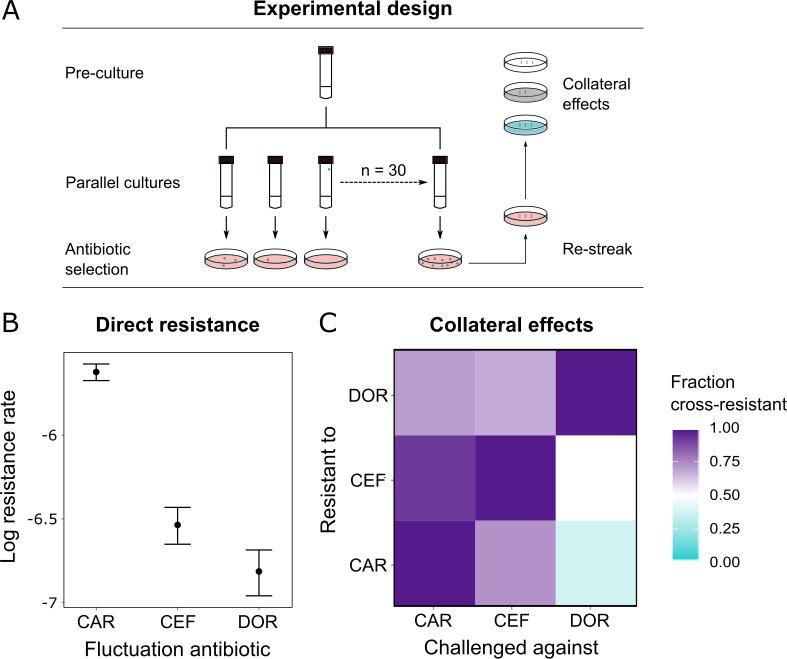
Since resistance to DOR was constrained in both the monotherapy and the switching treatments (Figure 2B), we hypothesized that DOR resistance was difficult to achieve compared to the other two β-lactams. Resistance against a given drug can arise because of spontaneous direct resistance and/or because of collateral resistance from the preceding antibiotics in the sequence. As a first step, we thus measured the spontaneous direct resistance rate with the classic fluctuation assay using identical inhibitory concentrations of the three antibiotics (Luria and Delbrück, 1943; Figure 4A, Supplementary file 1G). To determine the probability of indirect resistance in a second step, we isolated the obtained single-step mutants and quantified the fraction of cross-resistance towards the other two β-lactams with a patching assay (Figure 4A). We used a comparatively large number of spontaneous mutants for this analysis (n = 60 per antibiotic) to capture the stochastic nature of evolution and, in this context, the potential importance of collateral effects for bacterial adaptation, as previously emphasized (Nichol et al., 2019). We found that the spontaneous resistance rate was significantly lower for DOR than for CAR and CEF (likelihood ratio test, p<0.0001 and p<0.01, respectively; Supplementary file 1H, Figure 4B). Moreover, the resulting cross-resistance effects (Figure 4C) were particularly common towards CAR (93% of clones with spontaneous CEF resistance and 71% with DOR resistance) and CEF (73% of originally CAR-resistant clones and 67% DOR-resistant clones). By contrast, the smallest levels of cross-resistance were expressed towards DOR (36% of originally CAR-resistant clones and 50% CEF-resistant clones). The overall fraction of cross-resistant clones was significantly smaller towards DOR than either CEF or CAR (Fisher's exact test, p<0.0004; Supplementary file 1I). We conclude that of the three β-lactams DOR had the lowest probability for both direct and indirect resistance, thereby providing experimental support to the indication of constrained DOR resistance evolution obtained from the detailed phenotypic and genomic characterization of the evolved bacteria (Figure 2, Table 1).
Figure 4. Doripenem has the lowest rates of direct and indirect resistance.
(A) Schematic of the experimental protocol to determine spontaneous rates of resistance on each of the three β-lactams and the resulting collateral landscape. Briefly, an overnight culture was taken and split into 30 parallel cultures where bacteria were allowed to divide in the absence of an antibiotic and any other constraint. Spontaneous resistant mutants were selected on minimal inhibitory concentration (MIC) plates and restreaked to ensure genetic resistance. These mutants were then patched on MIC plates of the other two β-lactams to test for cross-resistance. (B) Comparison of rates of spontaneous resistance on the three β-lactams on a Log10 scale. Error bars depict CI95. All comparisons were found to be significantly different from each other (likelihood ratio test; CAR vs. CEF p<0.0001, CAR vs. DOR p<0.0001, and DOR vs. CEF p<0.01). (C) Landscape of collateral effects between the three β-lactams. Fraction of cross-resistant mutants per antibiotic combination is plotted. DOR has the least cases of cross-resistance of the three. A total of 60 mutants per antibiotic were used for collateral effect testing. The following supplementary material is available for Figure 4: Figure 4—source data 1, Supplementary file 1G–I. CAR: carbenicillin; CEF: cefsulodin; DOR: doripenem.
The rate of spontaneous resistance and resulting cross-resistance determine treatment efficacy
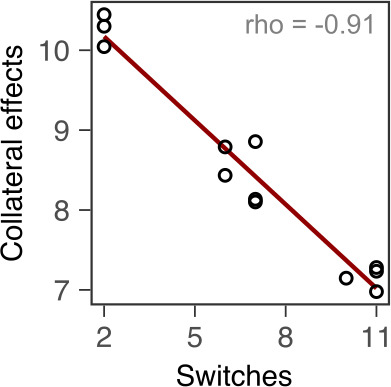
We used the collected information to identify the critical determinant(s) of treatment efficacy in the CAR-CEF-DOR triple β-lactam experiment. We assessed the influence of either the two experimental predictors (switching rate, temporal irregularity) or the three biological predictors (hysteresis, probability of spontaneous resistance, and resulting cross-resistance) on each of the evolutionary responses extinction, rate of growth adaptation, and multidrug resistance, using separate GLM-based analyses (see Materials and methods; Supplementary file 1J–O). For the biological predictors, we calculated the levels of cumulative hysteresis, cumulative probability of spontaneous resistance, and the cumulative levels of cross-resistance in each of the 16 individual treatments up to transfer 12 (see Materials and methods). We focused our analysis on the early phase of evolution up to transfer 12 as it appeared most critical for treatment efficacy, especially for population extinctions that usually occurred early (Figure 1E). Our analysis revealed that extinction was significantly associated with both the experimental predictors, switching rate (GLM, F = 14.44, p=0.0042, Figure 5B, Supplementary file 1J–M) and temporal irregularity (GLM, F = 10.53, p=0.0101, Supplementary file 1M). Temporal irregularity further showed a statistical trend with multidrug resistance (GLM, F = 4.19, p=0.0711, Supplementary file 1M). From our biological predictors, the cumulative cross-resistant fraction showed a significant association with extinction (GLM, F = 10.42, p=0.0121, Supplementary file 1O), while cumulative probability of spontaneous resistance showed a statistical trend (GLM, F = 4.14, p=0.0763, Supplementary file 1O). Indeed, the cumulative cross-resistant fraction and also the cumulative probability of spontaneous resistance are strongly correlated with extinction (Figure 5B). The cumulative cross-resistant fraction is also strongly correlated with switching rate (Figure 5—figure supplement 1), most likely explaining the latter impact on extinction. By contrast, cumulative hysteresis levels did not have a significant influence on any of the evolutionary responses (GLM, F = 0.16, p=0.7015, Supplementary file 1O). Taken together, our results suggest that in our sequential CAR-CEF-DOR treatments the switching rate, temporal irregularity of antibiotics, the probability of spontaneous resistance, and especially the resulting collateral effects (maximized by switching rate) determine treatment efficacy through their effect on bacterial extinction. The limiting factor appears to be constrained evolution of resistance and low levels of cross-resistance to DOR.
Figure 5. Bacterial extinction is correlated to switching rate, spontaneous rate of resistance, and spontaneous cross-resistance.
(A) Variation in experimental parameters, potential biological predictors, and the measured traits up to transfer 12. The experimental parameters include switching rate and regularity of change (high irregularity in dark). Potential biological predictors are cumulative levels of hysteresis (dark indicates protective effects), cumulative probabilities of spontaneous resistance (Spont. res., dark indicates higher probability), and cumulative level of collateral effects (Cross-res., dark indicates high fraction of cross-resistance). The evolutionary response was measured for population survival (max = 12), adaptation rate (Adapt. rate, n ≤ 12, extinct lineages excluded), evolved multidrug resistance (MDR) to treatment antibiotics CAR, DOR, and CEF (MDR, n = 16). (B) Variation in extinction was best explained by collateral effects between the antibiotics (for illustrative purposes, the red line depicts linear regression and ρS the Spearman’s rank correlation coefficient). The following supplementary material is available for Figure 5: Figure 5—figure supplement 1, Figure 5—source data 1, Supplementary file 1J–O. CAR: carbenicillin; CEF: cefsulodin; DOR: doripenem.
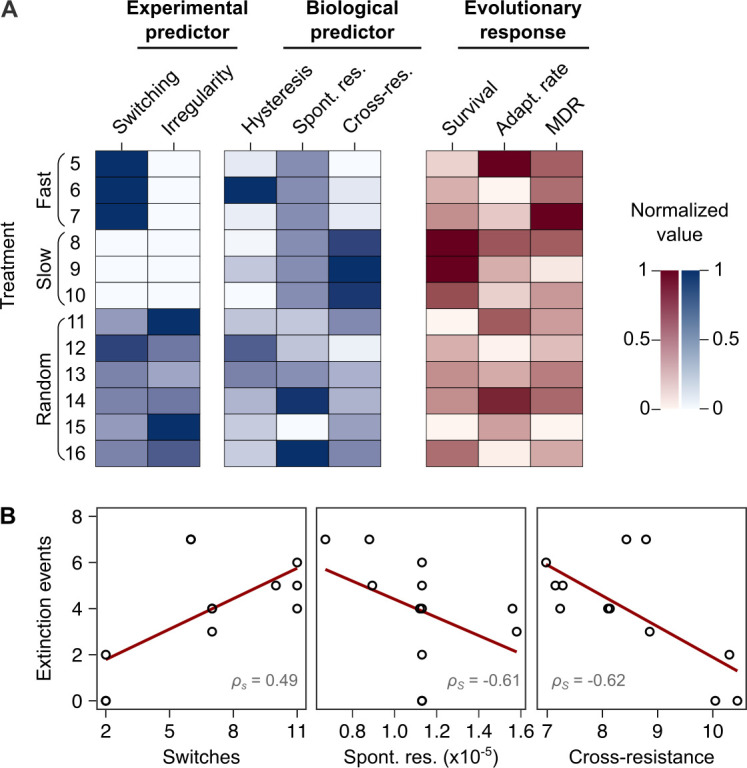
Figure 5—figure supplement 1. Correlation of switching rate with cumulative levels of collateral effects.
Discussion
Treatment with multiple β-lactam antibiotics is generally avoided due to the perceived fear of therapy failure from cross-resistance. Our work now challenges this widespread belief. We characterized the ability of replicate P. aeruginosa populations to evolve de novo resistance to sequential treatments with different drug sets. To our surprise, we found that sets of three β-lactams constrained bacterial adaptation by reducing bacterial survival. We demonstrate that treatment potency was determined by variation in the spontaneous rate of resistance to the β-lactams and the resulting collateral effects across sequential treatment protocols.
Our initial screen of sequential protocols with different antibiotic triplets revealed that the triple β-lactam sequences are at least as effective at causing extinction as sequences of antibiotics with distinct modes of actions. This finding is at first sight counterintuitive, but at second sight not completely unexpected. The joint application of two β-lactam drugs was in fact tested and found effective in a few previous studies (Rahme et al., 2014). For example, the β-lactam aztreonam was shown to interact synergistically with four other β-lactam drugs against multiple resistant isolates of Enterobacteriaceae and P. aeruginosa in vitro (Buesing and Jorgensen, 1984). A combination of ticarcillin with ceftazidime produced high efficacy in a rat peritonitis model (Shyu et al., 1987). In a treatment of bacterial soft tissue infections, the combination of cefotaxime and mecillinam led to higher clinical response rates than the tested monotherapy (File and Tan, 1983). Further, the dual β-lactam combination of ceftazidime plus piperacillin was as effective as the combination of ceftazidime and tobramycin in granulocytopenic cancer patients (Joshi et al., 1993). More recent studies demonstrated that a triple combination of meropenem, piperacillin, and tazobactam successfully constrained resistance evolution in Methicillin-resistant Staphylococcus aureus (MRSA), both in vitro and in a mouse model (Gonzales et al., 2015). In addition, the combination of cefotaxime and mecillinam was effective against Salmonella enterica harboring a mutant β-lactamase in a mouse model (Rosenkilde et al., 2019). Our findings add to the high potency of treatments with multiple β-lactams. We conclude that the use of multiple β-lactams, either as a combination or sequentially, is a commonly underappreciated form of therapy and its use opens new avenues to better utilize our existing antibiotic armamentarium.
Spontaneous rate of antibiotic resistance was found to play a critical role in the success of the CAR-CEF-DOR sequential treatment. The probability of spontaneous resistance on all three β-lactams was significantly different, with the rate of DOR resistance being the lowest. These rates determined the overall probability of acquiring direct resistance in treatment, which significantly correlated with the frequency of population extinction (Figure 5B). Resistance rates were previously shown to vary towards different antibiotics, for example, in Escherichia coli (El Meouche and Dunlop, 2018) and P. aeruginosa (Oliver et al., 2004). This variation can arise from genetic factors such as mutational target space and physiological factors like activation of the bacterial SOS response (Martinez and Baquero, 2000). Such information on resistance rates has so far been used for predicting the occurrence of resistance against single drugs, and antibiotics that target multiple pathways in a cell are considered advantageous in this context (Ross-Gillespie and Kümmerli, 2014). One example of the latter are compounds against S. aureus that inhibit both DNA gyrase and topoisomerase IV (Nyerges et al., 2020). The rate of resistance emergence may also be reduced by using adjuvants that target the SOS response (Bell and MacLean, 2018), as previously shown for compounds interfering with LexA activity leading to reduced resistance rates to ciprofloxacin and rifampicin in E. coli (Cirz et al., 2005). Our study extends the role of resistance rates of antibiotics beyond this convention. We show that inclusion of an antibiotic with relatively low spontaneous resistance emergence can enhance the potency of a sequential treatment design.
What could be the underlying reasons for the particular importance of DOR compared with the other β-lactams? DOR belongs to the carbapenem subclass of the β-lactam antibiotics. Carbapenems possess broad activity against Gram-positive and Gram-negative bacteria (Papp-Wallace et al., 2011) and are active against many β-lactamase-producing microbes since their thiazolidinic ring makes them relatively resistant to β-lactamase-mediated hydrolysis (Schafer et al., 2009). In contrast, the penicillin CAR is active mostly (albeit not exclusively) against Gram-negative bacteria (Castle, 2007) while the activity of the cephalosporin CEF is restricted to P. aeruginosa (Wright, 1986). Within P. aeruginosa, all three antibiotics show high potency against a large variety of clinical isolates (Castanheira et al., 2009; Neu and Scully, 1984; Traub and Raymond, 1970). Resistance rates for β-lactam antibiotics were assessed with different approaches across P. aeruginosa strains and clinical isolates, consistently showing that DOR has a particularly low propensity to select for resistance mutations, even when compared to other carbapenems (Barbosa et al., 2017; Barbosa et al., 2021; Sakyo et al., 2006; Tanimoto et al., 2008; Mushtaq et al., 2004; Fujimura et al., 2009). Therefore, the phenotype of reduced spontaneous resistance to DOR appears to be robustly expressed across different P. aeruginosa genotypes and does not extend to other carbapenems or β-lactams. One possible reason for this pattern may be variation in the range of β-lactam target proteins, in this case the penicillin binding proteins (PBPs), and where DOR is known to bind more of these PBPs than do CAR or CEF (Davies et al., 2008; Fontana et al., 2000; Rodriguez‐Tebár et al., 1982; Rodríguez-Tebar et al., 1982; Zapun et al., 2008; Zimmermann, 1980). Thus, target resistance to DOR would likely require a larger number of mutations than that to other β-lactams. Interestingly, another carbapenem, meropenem, targets the same PBPs as DOR (Davies et al., 2008) but has a higher resistance rate, suggesting that the underlying reasons for resistance rate variation are multifactorial. Taken together, effective resistance mutations against DOR seem to be less commonly available in P. aeruginosa in comparison to that against other drugs, including the here used CEF and CAR.
A key determinant of treatment potency was the reduced level of spontaneous cross-resistance to the sequentially applied drugs (Figure 5B). This effect was maximized by the switching rate (Figure 5—figure supplement 1). Our findings are consistent with the previously and repeatedly proposed importance of collateral sensitivity for the efficacy of sequential treatment protocols (Barbosa et al., 2019; Hernando-Amado et al., 2020; Imamovic and Sommer, 2013; Kim et al., 2014; Maltas and Wood, 2019; Yen and Papin, 2017). Even though we did not measure collateral sensitivity directly, the lack of cross-resistance is related as it indicates that the mutant cells, which have become resistant to one drug, maintain at least ancestral levels of susceptibility against the second drug. Moreover, our study focused on spontaneous emergence of cross-resistance (or lack thereof). By contrast, many previous studies established collateral effects after bacteria evolved resistance to the first drug over many generations, often followed by only a single antibiotic switch to assess the impact of collateral sensitivity on therapy success (Barbosa et al., 2019; Hernando-Amado et al., 2020; Imamovic and Sommer, 2013; Yen and Papin, 2017). Surprisingly, our study revealed potentially beneficial collateral effects between antibiotics of the same class. In fact, we chose these three β-lactams because our previous work demonstrated cross-resistance between most of them, although inferred upon multigenerational adaptation to the first drug (Barbosa et al., 2017). Our current finding of a lack of cross-resistance among some of these drugs now suggests that spontaneous mutants may have different collateral profiles than the lines, which adapted over many generations. Our results further suggest that the collateral effects of spontaneous mutants are likely to be more pertinent for the design of sequential treatments with fast switches among antibiotics. This suggestion is supported by two previous studies, in which the efficacy of fast sequential treatments was optimized by considering collateral effects for either single-step mutants of S. aureus, obtained after 20 hr exposure to three distinct antibiotics for 20 hr (Kim et al., 2014), or from Enterococcus faecalis populations adapted over 2 days to four distinct antibiotics (Maltas and Wood, 2019). As a side note, it is particularly interesting that our detailed resistance analysis consistently revealed almost all treatments to cause the evolution of collateral sensitivity towards the aminoglycoside gentamicin, but not the fluoroquinolone ciprofloxacin (Figure 2B, C), possibly indicating yet another treatment option – in cases where the applied triple β-lactam sequential protocols fail.
Temporal irregularity was additionally found to constrain bacterial adaptation. When bacteria experienced the antibiotics in an irregular pattern, this caused significantly increased extinction and to some degree reduced multidrug resistance. With CAR-DOR-CEF, the lowest multidrug resistance was observed in random sequential treatments (Figure 2—figure supplement 5), as also previously observed with CIP-GEN-CAR (Roemhild et al., 2018). Environmental change anticipation has been documented in several microorganisms (Mitchell et al., 2009; Mitchell and Pilpel, 2011), indicating their capability to specifically adapt to regular environmental change. Stochastic changes can make it harder to evolve anticipation (Roemhild and Schulenburg, 2019). Stochastic changes in environmental parameters were indeed found to constrain fitness in evolving bacteria (Hughes et al., 2007) and viruses (Alto et al., 2013). We show that irregular antibiotic sequences have potential to inhibit bacterial resistance evolution.
Unexpectedly, we further identified negative hysteresis for multiple combinations of the three β-lactams. However, cumulative hysteresis levels per treatment did not significantly associate with any of our measured evolutionary responses. In our previous study (Roemhild et al., 2018), within the CAR-CIP-GEN combination, negative hysteresis was expressed for the switches from CAR to GEN and CIP to GEN. Yet, only the CAR-GEN hysteresis was significantly associated to the evolutionary responses. Thus, hysteresis interactions can exist between antibiotics from the same or different classes, but they need not impact the evolutionary outcome of a sequential treatment protocol each time. In the current study, it appears that spontaneous resistance effects and the resulting cross-resistance effects are dominant over the β-lactam hysteresis. One potential explanation could be that insensitivity to β-lactam hysteresis evolves quickly. Nevertheless, it clearly warrants further research to assess whether negative hysteresis between the β-lactam drugs is robustly shown across strains of P. aeruginosa or other bacterial species and can somehow be exploited in sequential therapy, in analogy to the previous results with antibiotics from different classes (Roemhild et al., 2018).
Taken together, our study highlights that the available antibiotics offer unexplored, highly potent treatment options that can be harnessed to counter the spread of drug resistance. It further underscores the importance of evolutionary trade-offs such as reduced cross-resistance in treatment design and introduces spontaneous resistance rates of component antibiotics as a guiding principle for sequential treatments. It is ironic that the differential cross-resistance landscape of the β-lactams was a key factor contributing to treatment potency, even though the risk of cross-resistance is usually used to reject β-lactam-exclusive treatments. The underlying reasons for differential spontaneous and long-term cross-resistance between these drugs (including the underlying molecular mechanisms) are as yet unknown and clearly deserve further attention in the future. We conclude that a detailed understanding of both spontaneous resistance rates and resulting cross-resistances against different antibiotics should be of particular value to further improve the potency of sequential protocols.
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference |
Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Pseudomonas aeruginosa) | PA14 | https://doi.org/10.1126/science.7604262 | UCBPP-PA14 | |
| Chemical compound, drug | AZL (azlocillin) |
Sigma | A7926-1G | |
| Chemical compound, drug | CAR (carbenicillin) |
Carl Roth | 6344.2 | |
| Chemical compound, drug | CIP (ciprofloxacin) |
Sigma | 17850-5 G-F | |
| Chemical compound, drug | CEF (cefsulodin) |
Carl Roth | 4014.2 | |
| Chemical compound, drug | CEZ (ceftazidime) |
Sigma | C3809.1G | |
| Chemical compound, drug | DOR (doripenem) |
Sigma | 32138-25 MG | |
| Chemical compound, drug | GEN (gentamicin) |
Carl Roth | 2475.1 | |
| Chemical compound, drug | STR (streptomycin) |
Sigma | S6501-5 | |
| Chemical compound, drug | TIC (ticarcillin) |
Sigma | T5639-1G | |
| Software, algorithm | R: A language and environment for statistical computing | https://www.R-project.org/ |
Materials
All experiments were performed with P. aeruginosa UCBPP-PA14 (Rahme et al., 1995). Bacteria were grown in M9 minimal medium supplemented with glucose (2 g/L), citrate (0.58 g/L), and casamino acids (1 g/L) or on M9 minimal agar (1.5%) or Lysogeny broth (LB) agar. Antibiotics were added as indicated. Cultures and plates were incubated at 37°C. Experiments included biological replicates (initiated with independent clones of the bacteria, which were grown separately before the start of the experiment, or independent evolutionary lineages from the respective evolution treatments) and technical replicates (initiated from the same starting culture of the bacteria), as indicated below. For the experiments, treatment groups were run in parallel and randomized. Treatment names were masked in order to minimize observer bias.
Dose-response curves of ancestor
We used dose-response curves based on broth microdilution in order to determine antibiotic concentration causing inhibition level of 25% growth yield relative of untreated controls (inhibitory concentration 75 [IC75]) for the antibiotics azlocillin (AZL), carbenicillin (CAR), ciprofloxacin (CIP), cefsulodin (CEF), ceftazidime (CTZ), doripenem (DOR), gentamicin (GEN), and ticarcillin (TIC; see Supplementary file 1A for details on antibiotics). Briefly, bacteria were grown to exponential phase (OD600 = 0.08) and inoculated into 96-well plates (100 µL per well, 5 × 106 CFU/mL) containing linear concentration ranges close to MIC of the antibiotics in M9 medium. Antibiotic concentrations were randomized spatially. Bacteria were incubated for 12 hr after which optical density was measured in BioTek EON plate readers at 600 nm (OD600). We included six biological replicates and 1–2 technical replicates per concentration and antibiotic. Optical density was plotted against antibiotic concentration to obtain a dose-response curve. Model fitting was carried out using the package drc (Ritz et al., 2015) in the statistical environment R (R Development Core Team, 2020) and the fitted curve was used to predict IC75 values (Figure 1—figure supplement 1).
Evolution experiments
We carried out evolution experiments with the various combination of antibiotics according to the design described previously (Roemhild et al., 2018). A total of 16 treatments were included (Figure 1B). Treatments 1–4 were constant environments consisting of the monotherapy (#1–3) and no drug control (#4). Treatments 5–10 were the regular switching treatments. They switched between the antibiotics in a regular predictable fashion, either every transfer (fast; #5–7) or every fourth transfer (slow; #8–10). Treatments 11–16 consisted of the random treatments that switched fast in a temporally irregular fashion. The setup was designed to test the effect of switching rate and temporal irregularity.
Every treatment consisted of 12 replicate populations (initiated from six biological replicates × two technical replicates). All populations were started with an inoculum of 5 × 105 cells. Populations were propagated as 100 µL batch cultures in 96-well plates, with a transfer to fresh medium every 12 hr (transfer size 2% v/v). Antibiotic selection was applied at IC75 throughout. We monitored growth by OD600 measurements taken every 15 min through the entire evolution experiment (BioTek Instruments, USA; EON; 37°C, 180 rpm double-orbital shaking). Evolutionary growth dynamics were assessed by plotting the final OD achieved in every transfer (relative to final OD of no drug control; relative yield). Adaptation rate was calculated with a sliding window approach, where adaptation rate was the inverse of the transfer at which the mean relative yield of a sliding window of 12 transfers reached 0.75 for the first time. Cases of extinction were determined at the end of the experiment by counting wells in which no growth was observed after an additional incubation in antibiotic-free medium. Samples of the populations were frozen in regular intervals in 10% (v/v) DMSO and stored at −80°C for later analysis. The evolution experiments were carried out for a total of 96 transfers.
Resistance measurements of evolved populations
We characterized populations frozen at transfers 12 and 48 in detail because they represented the early and late phases of the evolution experiment. One population originating from a single biological replicate was chosen per treatment and plated onto LB agar. After incubation at 37°C, 20 colonies from each population were picked randomly and frozen in 10% (v/v) DMSO and stored at −80°C. These colonies, termed isolates, were considered to be representative biological replicates for each population. We constructed dose-response curves for the isolates using for each evolved population one technical replicate per isolate and four technical replicates of the ancestral PA14 strain, as described above, for the antibiotics CAR, CEF, DOR, GEN, and CIP. The integral of this curve for every isolate was calculated and the integral of the ancestral PA14 control subtracted. The resulting value was resistance of the isolate on the said antibiotic. We identified subpopulations in any given population by hierarchical clustering of the resistance profiles, as previously described (Roemhild et al., 2018). Resistance of a population was calculated by averaging the resistance of the isolates. Resistance of the population on CAR, CEF, and DOR was added to obtain a single value for multidrug resistance.
Whole-genome sequencing
From the frozen isolates at transfer 12, we chose three isolates per population (i.e., three biological replicates per population) for whole-genome sequencing to determine possible targets of selection. Each resistance cluster in the population was represented in the sequenced isolates. For the DOR monotherapy, isolates from transfer 48 were also sequenced as no phenotypic resistance was observed at transfer 12. Frozen isolates were thawed and grown in M9 medium at 37°C for 16–20 hr. We extracted DNA using a modified CTAB protocol (von der Schulenburg et al., 2001) and sequenced it at the Competence Centre for Genomic Analysis Kiel (CCGA Kiel; Institute for Clinical Microbiology, University Hospital Kiel), using Illumina Nextera DNA Flex library preparation and the MiSeq paired-end technology (2 × 300 bp). Quality control on the resulting raw reads was performed with FastQC (Andrews, 2010) and low-quality reads were trimmed using Trimmomatic (Bolger et al., 2014). We then used MarkDuplicates from the Picard Toolkit (http://broadinstitute.github.io/picard/) to remove duplicate reads and mapped the remaining reads to the P. aeruginosa UCBPP-PA14 genome (available at http://pseudomonas.com/strain/download) using Bowtie2 and samtools (Langmead and Salzberg, 2012; Li et al., 2009). Variant calling was done using the GATK suite (Poplin et al., 2018) and the called variants were annotated using SnpEFF (Cingolani et al., 2012) and the Pseudomonas Genome Database (https://www.pseudomonas.com/). We removed all variants that were detected in the no drug control as they likely represent adaptation to the medium and not the antibiotic. The fasta files of all sequenced isolates are available from NCBI under the BioProject number: PRJNA704789.
Hysteresis testing
The presence of cellular hysteresis was tested, following the previously developed protocol (Roemhild et al., 2018). Bacterial cells were grown to exponential phase (OD600 = 0.08), diluted 10-fold, and treated with IC75 of the first antibiotic. In the treatments where the pretreatment did not require an antibiotic, none was added. These cells were allowed to incubate for 15 min at 37°C and 150 rpm (pretreatment). After this, the first antibiotic was removed by centrifugation and fresh medium containing IC75 of a second antibiotic was added. In cases where the main treatment did not require an antibiotic, fresh medium without an antibiotic was added. Bacteria were now incubated for 8 hr at 37°C and 150 rpm (main treatment). Bacterial count was monitored through the main treatment by spotting assays. We used three biological replicates per treatment and, for CFU counting, four technical replicates per biological replicate and treatment. Log10 CFU/mL were plotted against time to obtain time-kill curves (Figure 3B). The level of hysteresis was calculated as the difference between the antibiotic switch and only main treatment curves.
Agar dilution
We determined the MIC on M9 agar for the antibiotics CAR, CEF, and DOR according to the EUCAST protocol (https://doi.org/10.1046/j.1469-0691.2000.00142.x) that was modified to account for inoculum effect in our fluctuation assay setup. UCBPP-PA14 was grown in M9 medium at 37°C for 20 hr. 5 × 105 cells were taken from the stationary phase cultures and spread on M9 agar plates containing doubling dilutions of the antibiotic. Plates were incubated at 37°C for 20–24 hr. MIC was read as the lowest concentration at which no growth of bacteria was seen. MIC determination for each antibiotic was done for three biological replicates (no additional technical replication).
Fluctuation assay
We measured resistance rates on the three β-lactams using the classic fluctuation assay (Luria and Delbrück, 1943). Briefly, a single colony of UCBPP-PA14 was inoculated to 10 mL M9 and incubated at 37°C, 150 rpm for 20 hr. This primary culture was used to start 30 parallel cultures all having a starting concentration of 102 CFU/mL. The parallel cultures were considered biological replicates and incubated at 37°C, 150 rpm for 20 hr. Thereafter, 5 × 105 cells were plated onto MIC plates of CAR, CEF, and DOR. The plates were incubated for 40 hr at 37°C. The resulting mutant colonies were taken and patched on identical antibiotic MIC plates to ensure genetic resistance. Colonies that grew after patching were counted. We used counts from all 30 cultures to estimate resistance rate on each antibiotic using the package rSalvador (Zheng, 2017) in R (R Development Core Team, 2020).
Patching assay
We assessed the extent of cross-resistance associated with each β-lactam using the mutants obtained from the fluctuation assay. Sixty mutants with genetic resistance to a given β-lactam were considered biological replicates and patched onto MIC plates of the two other β-lactams. The patched plates were incubated for 16–20 hr at 37°C. If the mutant grew at MIC of the second β-lactam, it was counted as resistant. If it did not grow at the MIC of the second β-lactam, it was counted as susceptible. For each switch between two drugs, the fraction of cross-resistant mutants was calculated as
Statistical analysis for cross-resistance on secondary antibiotic
To test whether the secondary antibiotic had an influence on the degree of cross-resistance of the mutants obtained from the fluctuation assay, we conducted a Fischer’s exact test followed by post hoc comparisons using the R package rcompanion (Mangiafico, 2016). The obtained p-values were then corrected for multiple testing using false discovery rate.
Statistical analysis of adaptive growth dynamics
To test whether main treatment types were associated with altered dynamics of adaptation in non-extinct populations, we analyzed the trajectories of relative growth yield (as plotted in Figure 1E and Figure 2A) of drug-treated populations using a GLM, including sequence (##1–16) and transfer as fixed factors and preculture and replicate population as nested random factors (see Supplementary file 1B for details). Comparisons between main treatment groups were performed using pairwise post hoc tests and z statistics. All p-values were corrected for multiple testing using false discovery rate. The analysis was performed separately for the three time phases ‘early’ (transfers 2–12), ‘middle’ (transfers 13–48), and ‘late’ (transfers 49–96) of the experiment, thus fulfilling the model assumption of response linearity. All statistical analyses were carried out in the statistical environment R (R Development Core Team, 2020).
Statistical analysis of evolved multidrug β-lactam resistance
To test whether evolved populations displayed distinct multidrug β-lactam resistance depending on their main treatment type, we analyzed multidrug β-lactam resistance of evolved isolates – the sum of resistance values against CAR, CEF, and DOR (as plotted in Figure 3B, C) – using a GLM. The model included sequence (##1–16) as fixed factor and replicate population as nested random factor (see Supplementary file 1C for detailed information). Comparisons between main treatment groups were performed using pairwise post hoc tests and z statistics. All p-values were corrected for multiple testing using false discovery rate. The analysis was performed separately for the ‘early’ (after transfers 12) and ‘middle’ (transfer 48) time points of the evolution experiment using the R statistical environment (R Development Core Team, 2020).
Statistical analysis of treatment potency predictors
To test whether our experimental (switching rate and temporal irregularity) and biological predictors (hysteresis, probability of direct resistance, and cross effects) were able to explain the variability in our evolutionary responses (extinction, rate of growth adaptation, and multidrug resistance) we carried out a GLM analysis. Values per treatment protocol for the biological predictors were calculated and the GLM analysis then carried out in R (R Development Core Team, 2020). We used the lm and anova commands and the main effects model: response ~ switching rate + irregularity for the experimental predictors and response ~ hysteresis + spontaneous resistance + mutant fraction cross-resistant for the biological predictors.
Acknowledgements
We would like to thank Leif Tueffers and João Botelho for discussions and suggestions as well as Kira Haas and Julia Bunk for technical support. We acknowledge financial support from the German Science Foundation (grant SCHU 1415/12-2 to HS, and funding under Germany’s Excellence Strategy EXC 2167-390884018 as well as the Research Training Group 2501 TransEvo to HS and SN), the Max-Planck Society (IMPRS scholarship to AB; Max-Planck fellowship to HS), and the Leibniz Science Campus Evolutionary Medicine of the Lung (EvoLUNG, to HS and SN). This work was further supported by the German Science Foundation Research Infrastructure NGS_CC (project 407495230) as part of the Next Generation Sequencing Competence Network (project 423957469). NGS analyses were carried out at the Competence Centre for Genomic Analysis Kiel (CCGA Kiel).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Hinrich Schulenburg, Email: hschulenburg@zoologie.uni-kiel.de.
Vaughn S Cooper, University of Pittsburgh, United States.
George H Perry, Pennsylvania State University, United States.
Funding Information
This paper was supported by the following grants:
Deutsche Forschungsgemeinschaft SCHU 1415/12-2 to Hinrich Schulenburg.
Deutsche Forschungsgemeinschaft EXC 2167-390884018 to Stefan Niemann, Hinrich Schulenburg.
Deutsche Forschungsgemeinschaft GRK 2501 to Stefan Niemann, Hinrich Schulenburg.
Max-Planck-Gesellschaft IMPRS Stipend to Aditi Batra.
Max-Planck-Gesellschaft Fellowship to Hinrich Schulenburg.
Leibniz-Gemeinschaft EvoLUNG to Stefan Niemann, Hinrich Schulenburg.
Deutsche Forschungsgemeinschaft 407495230 to Sören Franzenburg.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing - original draft, Writing - review and editing.
Conceptualization, Data curation, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing - original draft, Writing - review and editing.
Investigation, Methodology, Writing - review and editing.
Investigation, Project administration, Whole genome sequencing.
Supervision, Project administration, Writing - review and editing.
Conceptualization, Formal analysis, Supervision, Funding acquisition, Writing - original draft, Project administration, Writing - review and editing.
Additional files
(A) List of antibiotics used for the evolution experiments. (B) Statistical analysis of main evolution treatments for the evolutionary dynamics shown in Figure 2A. (C) Statistical analysis of main evolution treatments for the multidrug β-lactam resistance after transfer 12 in Figure 2B. (D) Statistical analysis of main evolution treatments for the multidrug β-lactam resistance after transfer 48 in Figure 2C. (E) Statistical analysis of main evolution treatments for Shannon diversity in Figure 2B, C. (F) Comparison of resistance profiles between transfer 12 and transfer 48 for Figure 2—figure supplement 4 using Wilcoxon test and Bonferroni correctiona. (G) Minimum inhibitory concentrations (MICs) as determined by agar dilution for the three β-lactams and as used for the fluctuation assays shown in Figure 4. (H) Likelihood ratio test to assess pairwise variation in spontaneous resistance rates for the three β-lactam antibiotics shown in Figure 4B. (I) Post hoc comparisons based on the false discovery rate for phenotype of cross-resistance on secondary antibiotic as shown in Figure 4C. (J) Analysis of variance of the consequences of the main treatment type on the three measured responses for transfer 12 of the triple β-lactam experiment as summarized in Figure 5. (K) Post hoc comparison based on the false discovery rate of the effect of the main treatment types on extinction for the triple β-lactam experiment as summarized in Figure 5. (L) General linear model analysis of the consequences of the experimental predictors switching rate and irregularity on the three measured responses for transfer 12 of the triple β-lactam experiment as summarized in Figure 5. (M) Main effect tests for the consequences of switching rate and irregularity on extinction and multidrug resistance (MDR) for transfer 12 of the triple β-lactam evolution experiment as summarized in Figure 5. (N) General linear model analysis of the consequences of the three considered biological predictors, cumulative probability of spontaneous resistance, cross-resistance, and hysteresis on the three measured responses for transfer 12 of the triple β-lactam experiment as summarized in Figure 5. (O) Main effect tests for the consequences of the three considered biological predictors, cumulative probability of spontaneous resistance, cross-resistance, and hysteresis on extinction of the triple β-lactam evolution experiment as summarized in Figure 5.
Data availability
Sequencing data have been deposited at NCBI under the BioProject number: PRJNA704789. All other data is provided in the supplementary source data files.
The following dataset was generated:
Batra A. 2021. Sequential beta-lactam treatment genomics. NCBI BioProject. PRJNA704789
References
- Alto BW, Wasik BR, Morales NM, Turner PE. Stochastic temperatures impede rna virus adaptation. Evolution. 2013;67:969–979. doi: 10.1111/evo.12034. [DOI] [PubMed] [Google Scholar]
- Andersson DI, Balaban NQ, Baquero F, Courvalin P, Glaser P, Gophna U, Kishony R, Molin S, Tønjum T. Antibiotic resistance: turning evolutionary principles into clinical reality. FEMS Microbiology Reviews. 2020;44:171–188. doi: 10.1093/femsre/fuaa001. [DOI] [PubMed] [Google Scholar]
- Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 0.11.9Babraham Bioinformatics. 2010 https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- Band VI, Hufnagel DA, Jaggavarapu S, Sherman EX, Wozniak JE, Satola SW, Farley MM, Jacob JT, Burd EM, Weiss DS. Antibiotic combinations that exploit heteroresistance to multiple drugs effectively control infection. Nature Microbiology. 2019;4:1627–1635. doi: 10.1038/s41564-019-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa C, Trebosc V, Kemmer C, Rosenstiel P, Beardmore R, Schulenburg H, Jansen G. Alternative evolutionary paths to bacterial antibiotic resistance cause distinct collateral effects. Molecular Biology and Evolution. 2017;34:2229–2244. doi: 10.1093/molbev/msx158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa C, Beardmore R, Schulenburg H, Jansen G. Antibiotic combination efficacy (ACE) networks for a Pseudomonas aeruginosa model. PLOS Biology. 2018;16:e2004356. doi: 10.1371/journal.pbio.2004356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa C, Römhild R, Rosenstiel P, Schulenburg H. Evolutionary stability of collateral sensitivity to antibiotics in the model pathogen Pseudomonas aeruginosa. eLife. 2019;8:e51481. doi: 10.7554/eLife.51481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa C, Mahrt N, Bunk J, Graßer M, Rosenstiel P, Jansen G, Schulenburg H. The genomic basis of rapid adaptation to antibiotic combination therapy in Pseudomonas aeruginosa. Molecular Biology and Evolution. 2021;38:449–464. doi: 10.1093/molbev/msaa233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baym M, Stone LK, Kishony R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science. 2016;351:aad3292. doi: 10.1126/science.aad3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G, MacLean C. The search for 'Evolution-Proof' Antibiotics. Trends in Microbiology. 2018;26:471–483. doi: 10.1016/j.tim.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Bloemberg GV, Keller PM, Stucki D, Trauner A, Borrell S, Latshang T, Coscolla M, Rothe T, Hömke R, Ritter C, Feldmann J, Schulthess B, Gagneux S, Böttger EC. Acquired resistance to bedaquiline and delamanid in therapy for tuberculosis. New England Journal of Medicine. 2015;373:1986–1988. doi: 10.1056/NEJMc1505196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buesing MA, Jorgensen JH. In vitro activity of aztreonam in combination with newer beta-lactams and amikacin against multiply resistant gram-negative bacilli. Antimicrobial Agents and Chemotherapy. 1984;25:283–285. doi: 10.1128/AAC.25.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot G, Florit-Mendoza L, Sánchez-Diener I, Zamorano L, Oliver A. Deciphering β-lactamase-independent β-lactam resistance evolution trajectories in Pseudomonas aeruginosa. The Journal of Antimicrobial Chemotherapy. 2018;73:3322–3331. doi: 10.1093/jac/dky364. [DOI] [PubMed] [Google Scholar]
- Castanheira M, Jones RN, Livermore DM. Antimicrobial activities of doripenem and other carbapenems against Pseudomonas aeruginosa, other nonfermentative bacilli, and Aeromonas spp. Diagnostic Microbiology and Infectious Disease. 2009;63:426–433. doi: 10.1016/j.diagmicrobio.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Castle SS. In: XPharm: The Comprehensive Pharmacology Reference. Enna S. J, Bylund D. B, editors. New York: Elsevier; 2007. Carbenicillin; pp. 1–5. [DOI] [Google Scholar]
- Chait R, Craney A, Kishony R. Antibiotic interactions that select against resistance. Nature. 2007;446:668–671. doi: 10.1038/nature05685. [DOI] [PubMed] [Google Scholar]
- Cingolani P, Platts A, Wang leL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. A program for annotating and predicting the effects of single Nucleotide Polymorphisms, SnpEff: snps in the genome of Drosophila Melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirz RT, Chin JK, Andes DR, de Crécy-Lagard V, Craig WA, Romesberg FE. Inhibition of mutation and combating the evolution of antibiotic resistance. PLOS Biology. 2005;3:e176. doi: 10.1371/journal.pbio.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TA, Shang W, Bush K, Flamm RK. Affinity of Doripenem and Comparators to Penicillin-Binding Proteins in Escherichia coli and Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy. 2008;52:1510–1512. doi: 10.1128/AAC.01529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Meouche I, Dunlop MJ. Heterogeneity in efflux pump expression predisposes antibiotic-resistant cells to mutation. Science. 2018;362:686–690. doi: 10.1126/science.aar7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File TM, Tan JS. Amdinocillin plus cefoxitin versus cefoxitin alone in therapy of mixed soft tissue infections (including diabetic foot infections) The American Journal of Medicine. 1983;75:100–105. doi: 10.1016/0002-9343(83)90103-1. [DOI] [PubMed] [Google Scholar]
- Fontana R, Cornaglia G, Ligozzi M, Mazzariol A. The final goal: penicillin-binding proteins and the target of cephalosporins. Clinical Microbiology and Infection. 2000;6:34–40. doi: 10.1111/j.1469-0691.2000.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Fuentes-Hernandez A, Plucain J, Gori F, Pena-Miller R, Reding C, Jansen G, Schulenburg H, Gudelj I, Beardmore R. Using a sequential regimen to eliminate Bacteria at sublethal antibiotic dosages. PLOS Biology. 2015;13:e1002104. doi: 10.1371/journal.pbio.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T, Anan N, Sugimori G, Watanabe T, Jinushi Y, Yoshida I, Yamano Y. Susceptibility of Pseudomonas aeruginosa clinical isolates in Japan to doripenem and other antipseudomonal agents. International Journal of Antimicrobial Agents. 2009;34:523–528. doi: 10.1016/j.ijantimicag.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Gonzales PR, Pesesky MW, Bouley R, Ballard A, Biddy BA, Suckow MA, Wolter WR, Schroeder VA, Burnham C-AD, Mobashery S, Chang M, Dantas G. Synergistic, collaterally sensitive β-lactam combinations suppress resistance in MRSA. Nature Chemical Biology. 2015;11:855–861. doi: 10.1038/nchembio.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AR, Kang PJ, Engel JN. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Molecular Microbiology. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- Hernando-Amado S, Sanz-García F, Martínez JL. Rapid and robust evolution of collateral sensitivity in Pseudomonas aeruginosa antibiotic-resistant mutants. Science Advances. 2020;6:eaba5493. doi: 10.1126/sciadv.aba5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjort K, Jurén P, Toro JC, Hoffner S, Andersson DI, Sandegren L. Dynamics of Extensive Drug Resistance Evolution of Mycobacterium tuberculosis in a Single Patient During 9 Years of Disease and Treatment. The Journal of Infectious Diseases. 2020;5:jiaa625. doi: 10.1093/infdis/jiaa625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes BS, Cullum AJ, Bennett AF. An experimental evolutionary study on adaptation to temporally fluctuating pH in Escherichia coli. Physiological and Biochemical Zoology. 2007;80:406–421. doi: 10.1086/518353. [DOI] [PubMed] [Google Scholar]
- Imamovic L, Sommer MOA. Use of collateral sensitivity networks to design drug cycling protocols that avoid resistance development. Science Translational Medicine. 2013;5:204ra132. doi: 10.1126/scitranslmed.3006609. [DOI] [PubMed] [Google Scholar]
- Joshi JH, Newman KA, Brown BW, Finley RS, Ruxer RL, Moody MA, Schimpff SC. Double ?-lactam regimen compared to an aminoglycoside/?-lactam regimen as empiric antibiotic therapy for febrile granulocytopenic cancer patients. Supportive Care in Cancer. 1993;1:186–194. doi: 10.1007/BF00366445. [DOI] [PubMed] [Google Scholar]
- Kim S, Lieberman TD, Kishony R. Alternating antibiotic treatments constrain evolutionary paths to multidrug resistance. PNAS. 2014;111:14494–14499. doi: 10.1073/pnas.1409800111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O. Antibiotic resistance—the need for global solutions. The Lancet Infectious Diseases. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- Lázár V, Pal Singh G, Spohn R, Nagy I, Horváth B, Hrtyan M, Busa‐Fekete R, Bogos B, Méhi O, Csörgő B, Pósfai G, Fekete G, Szappanos B, Kégl B, Papp B, Pál C. Bacterial evolution of antibiotic hypersensitivity. Molecular Systems Biology. 2013;9:700. doi: 10.1038/msb.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin-Reisman I, Ronin I, Gefen O, Braniss I, Shoresh N, Balaban NQ. Antibiotic tolerance facilitates the evolution of resistance. Science. 2017;355:826–830. doi: 10.1126/science.aaj2191. [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup The sequence alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Hancock RE. Cloning and characterization of the Pseudomonas aeruginosa pbpB gene encoding penicillin-binding protein 3. Antimicrobial Agents and Chemotherapy. 1995;39:1871–1874. doi: 10.1128/AAC.39.8.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore DM. beta-Lactamases in laboratory and clinical resistance. Clinical Microbiology Reviews. 1995;8:557–584. doi: 10.1128/CMR.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria SE, Delbrück M. Mutations of Bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltas J, Wood KB. Pervasive and diverse collateral sensitivity profiles inform optimal strategies to limit antibiotic resistance. PLOS Biology. 2019;17:e3000515. doi: 10.1371/journal.pbio.3000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiafico SS. Summar and analysis of extension program evaluation in R. 1.18.8Rutgers. 2016 https://scholar.google.com/citations?user=W9CxPFIAAAAJ&hl=en
- Martinez JL, Baquero F. Mutation frequencies and antibiotic resistance. Antimicrobial Agents and Chemotherapy. 2000;44:1771–1777. doi: 10.1128/AAC.44.7.1771-1777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker M, Tueffers L, Vallier M, Groth EE, Sonnenkalb L, Unterweger D, Baines JF, Niemann S, Schulenburg H. Evolutionary approaches to combat antibiotic resistance: opportunities and challenges for precision medicine. Frontiers in Immunology. 2020;11:1938. doi: 10.3389/fimmu.2020.01938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A, Romano GH, Groisman B, Yona A, Dekel E, Kupiec M, Dahan O, Pilpel Y. Adaptive prediction of environmental changes by microorganisms. Nature. 2009;460:220–224. doi: 10.1038/nature08112. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Pilpel Y. A mathematical model for adaptive prediction of environmental changes by microorganisms. PNAS. 2011;108:7271–7276. doi: 10.1073/pnas.1019754108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq S, Ge Y, Livermore DM. Doripenem versus Pseudomonas aeruginosa In Vitro: Activity against Characterized Isolates, Mutants, and Transconjugants and Resistance Selection Potential. Antimicrobial Agents and Chemotherapy. 2004;48:3086–3092. doi: 10.1128/AAC.48.8.3086-3092.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu HC, Scully BE. Activity of cefsulodin and other agents against Pseudomonas aeruginosa. Clinical Infectious Diseases. 1984;6:S667–S677. doi: 10.1093/clinids/6.Supplement_3.S667. [DOI] [PubMed] [Google Scholar]
- Nichol D, Rutter J, Bryant C, Hujer AM, Lek S, Adams MD, Jeavons P, Anderson ARA, Bonomo RA, Scott JG. Antibiotic collateral sensitivity is contingent on the repeatability of evolution. Nature Communications. 2019;10:334. doi: 10.1038/s41467-018-08098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyerges A, Tomašič T, Durcik M, Revesz T, Szili P, Draskovits G, Bogar F, Skok Žiga, Zidar N, Ilaš J, Zega A, Kikelj D, Daruka L, Kintses B, Vasarhelyi B, Foldesi I, Kata D, Welin M, Kimbung R, Focht D, Mašič LP, Pal C. Rational design of balanced dual-targeting antibiotics with limited resistance. PLOS Biology. 2020;18:e3000819. doi: 10.1371/journal.pbio.3000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver A, Levin BR, Juan C, Baquero F, Blázquez J, Blázquez J. Hypermutation and the Preexistence of Antibiotic-Resistant Pseudomonas aeruginosa Mutants: Implications for Susceptibility Testing and Treatment of Chronic Infections. Antimicrobial Agents and Chemotherapy. 2004;48:4226–4233. doi: 10.1128/AAC.48.11.4226-4233.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrobial Agents and Chemotherapy. 2011;55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Miller R, Laehnemann D, Jansen G, Fuentes-Hernandez A, Rosenstiel P, Schulenburg H, Beardmore R. When the most potent combination of antibiotics selects for the greatest bacterial load: the Smile-Frown transition. PLOS Biology. 2013;11:e1001540. doi: 10.1371/journal.pbio.1001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplin R, Ruano-Rubio V, DePristo MA, Fennell TJ, Carneiro MO, der A, Kling DE, Gauthier LD, Levy-Moonshine A, Roazen D, Shakir K, Thibault J, Chandran S, Whelan C, Lek M, Gabriel S, Daly MJ, Neale B, MacArthur DG, Banks E. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv. 2018 doi: 10.1101/201178. [DOI]
- R Development Core Team . Vienna, Austria: R Foundation for Statistical Computing; 2020. https://www.R-project.org/ [Google Scholar]
- Rahme L, Stevens E, Wolfort S, Shao J, Tompkins R, Ausubel F. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- Rahme C, Butterfield JM, Nicasio AM, Lodise TP. Dual beta-lactam therapy for serious Gram-negative infections: is it time to revisit? Diagnostic Microbiology and Infectious Disease. 2014;80:239–259. doi: 10.1016/j.diagmicrobio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Ritz C, Baty F, Streibig JC, Gerhard D. Dose-Response analysis using R. PLOS ONE. 2015;10:e0146021. doi: 10.1371/journal.pone.0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Tebar A, Rojo F, Dámaso D, Vázquez D. Carbenicillin resistance of Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy. 1982;22:255–261. doi: 10.1128/AAC.22.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Tebár A, Rojo F, Montilla JC, Vázquez D. Interaction of β-lactam antibiotics with penicillin-binding proteins from Pseudomonas aeruginosa. FEMS Microbiology Letters. 1982;14:295–298. doi: 10.1111/j.1574-6968.1982.tb00016.x. [DOI] [Google Scholar]
- Roemhild R, Barbosa C, Beardmore RE, Jansen G, Schulenburg H. Temporal variation in antibiotic environments slows down resistance evolution in pathogenic Pseudomonas aeruginosa. Evolutionary Applications. 2015;8:945–955. doi: 10.1111/eva.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemhild R, Gokhale CS, Dirksen P, Blake C, Rosenstiel P, Traulsen A, Andersson DI, Schulenburg H. Cellular hysteresis as a principle to maximize the efficacy of antibiotic therapy. PNAS. 2018;115:9767–9772. doi: 10.1073/pnas.1810004115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemhild R, Schulenburg H. Evolutionary ecology meets the antibiotic crisis: can we control pathogen adaptation through sequential therapy? Evolution, Medicine, and Public Health. 2019;2019:37–45. doi: 10.1093/emph/eoz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkilde CEH, Munck C, Porse A, Linkevicius M, Andersson DI, Sommer MOA. Collateral sensitivity constrains resistance evolution of the CTX-M-15 β-lactamase. Nature Communications. 2019;10:618. doi: 10.1038/s41467-019-08529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Gillespie A, Kümmerli R. 'Evolution-proofing' antibacterials. Evolution, Medicine, and Public Health. 2014;2014:134–135. doi: 10.1093/emph/eou020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakyo S, Tomita H, Tanimoto K, Fujimoto S, Ike Y. Potency of carbapenems for the prevention of Carbapenem-Resistant mutants of Pseudomonas aeruginosa: the high potency of a new carbapenem doripenem. J Antibiot. 2006;59:220–228. doi: 10.1038/ja.2006.31. [DOI] [PubMed] [Google Scholar]
- Sanz-García F, Hernando-Amado S, Martínez JL. Mutation-Driven evolution of Pseudomonas aeruginosa in the presence of either ceftazidime or Ceftazidime-Avibactam. Antimicrobial Agents and Chemotherapy. 2018;62:e01379-18. doi: 10.1128/AAC.01379-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J, Goff D, Mangino J. Doripenem: a new addition to the carbapenem class of antimicrobials. Recent Patents Anti-Infect Drug Disc. 2009;4:18–28. doi: 10.2174/157489109787236283. [DOI] [PubMed] [Google Scholar]
- Shyu WC, Nightingale CH, Quintiliani R. Pseudomonas peritonitis in neutropenic rats treated with amikacin, ceftazidime and ticarcillin, alone and in combination. Journal of Antimicrobial Chemotherapy. 1987;19:807–814. doi: 10.1093/jac/19.6.807. [DOI] [PubMed] [Google Scholar]
- Sobel ML, Hocquet D, Cao L, Plesiat P, Poole K. Mutations in PA3574 ( nalD ) Lead to Increased MexAB-OprM Expression and Multidrug Resistance in Laboratory and Clinical Isolates of Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy. 2005;49:1782–1786. doi: 10.1128/AAC.49.5.1782-1786.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szybalski W, Bryson V. Genetic studies on microbial cross resistance to toxic agents I. Journal of Bacteriology. 1952;64:489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto K, Tomita H, Fujimoto S, Okuzumi K, Ike Y. Fluoroquinolone Enhances the Mutation Frequency for Meropenem-Selected Carbapenem Resistance in Pseudomonas aeruginosa , but Use of the High-Potency Drug Doripenem Inhibits Mutant Formation. Antimicrobial Agents and Chemotherapy. 2008;52:3795–3800. doi: 10.1128/AAC.00464-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub WH, Raymond EA. Susceptibility of Pseudomonas aeruginosa to carbenicillin. Applied Microbiology. 1970;20:630–632. doi: 10.1128/am.20.4.630-632.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tueffers L, Barbosa C, Bobis I, Schubert S, Höppner M, Rühlemann M, Franke A, Rosenstiel P, Friedrichs A, Krenz-Weinreich A, Fickenscher H, Bewig B, Schreiber S, Schulenburg H. Pseudomonas aeruginosa populations in the cystic fibrosis lung lose susceptibility to newly applied β-lactams within 3 days. Journal of Antimicrobial Chemotherapy. 2019;74:2916–2925. doi: 10.1093/jac/dkz297. [DOI] [PubMed] [Google Scholar]
- von der Schulenburg JH, Hancock JM, Pagnamenta A, Sloggett JJ, Majerus ME, Hurst GD. Extreme length and length variation in the first ribosomal internal transcribed spacer of ladybird beetles (Coleoptera: coccinellidae) Molecular Biology and Evolution. 2001;18:648–660. doi: 10.1093/oxfordjournals.molbev.a003845. [DOI] [PubMed] [Google Scholar]
- Walsh C. Antibiotics: Actions, Origins, Resistance. Washington: ASM Media & PR; 2003. [Google Scholar]
- Woods RJ, Read AF. Clinical management of resistance evolution in a bacterial infection. Evolution, Medicine, and Public Health. 2015;2015:281–288. doi: 10.1093/emph/eov025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DB. Cefsulodin. Drug Intelligence & Clinical Pharmacy. 1986;20:845–849. doi: 10.1177/106002808602001104. [DOI] [PubMed] [Google Scholar]
- Yen P, Papin JA. History of antibiotic adaptation influences microbial evolutionary dynamics during subsequent treatment. PLOS Biology. 2017;15:e2001586. doi: 10.1371/journal.pbio.2001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Reyes SG, Tsuda S, Horinouchi T, Furusawa C, Cronin L. Time-programmable drug dosing allows the manipulation, suppression and reversal of antibiotic drug resistance in vitro. Nature Communications. 2017;8:15589. doi: 10.1038/ncomms15589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapun A, Contreras-Martel C, Vernet T. Penicillin-binding proteins and β-lactam resistance. FEMS Microbiology Reviews. 2008;32:361–385. doi: 10.1111/j.1574-6976.2007.00095.x. [DOI] [PubMed] [Google Scholar]
- Zheng Q. rSalvador: an R package for the fluctuation experiment. G3: Genes, Genomes, Genetics. 2017;7:3849–3856. doi: 10.1534/g3.117.300120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Barbosa C, Woods RJ. Why is preventing antibiotic resistance so hard? Analysis of failed resistance management. Evolution, Medicine, and Public Health. 2020;2020:102–108. doi: 10.1093/emph/eoaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W. Penetration of beta-lactam antibiotics into their target enzymes in Pseudomonas aeruginosa: comparison of a highly sensitive mutant with its parent strain. Antimicrobial Agents and Chemotherapy. 1980;18:94–100. doi: 10.1128/AAC.18.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]