Scheme 8.
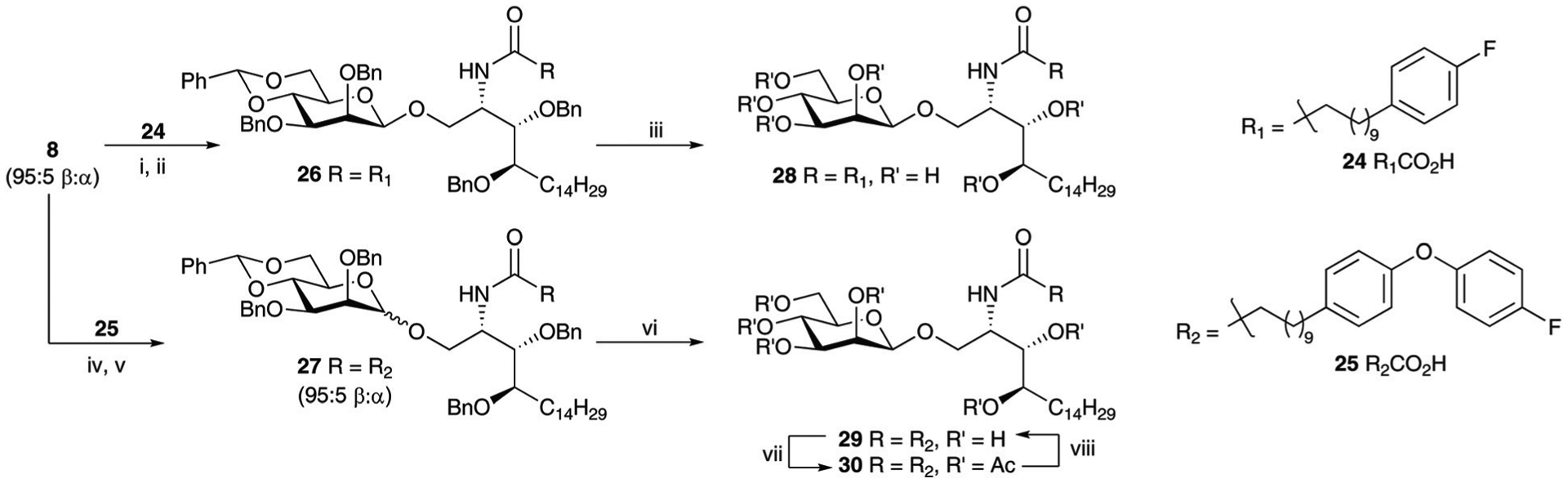
Reagents and conditions; (i) (a) Me3P (1 M in THF), anhyd. THF, 0 °C to rt, 5 h; (b) NaOH (2 M), o/n, 18 quant; (ii) (a) EDCI·HCl, HOBt, 10 : 4 CH2Cl2 : DMF, rt, 30 min; then 18, DIPEA, anhyd. CH2Cl2, rt, o/n; β−26 70% over two steps; (iii) Pd/C, H2, rt, o/n; 28 83% (β-only); (iv) (a) Me3P (1 M in THF), anhyd. THF, 0 °C to rt, 5 h; (b) NaOH (2 M), o/n, 18 quant; (v) (a) EDCI·HCl, HOBt, 10 : 4 CH2Cl2 : DMF, rt, 30 min; then 18, DIPEA, anhyd. CH2Cl2, rt, o/n; 27 – α : β 5 : 95, 65% over two steps; (vi) Pd/C, H2, rt, o/n; 29 94% (mixture of anomers); (vii) Ac2O, py, rt, o/n β−30 74%; (viii) 2.5 M NaOMe, MeOH, rt, 3 h.
