Abstract
Background & Aims
While direct oral anticoagulants (DOACs) are increasingly used in patients with liver disease, safety data especially in advanced chronic liver disease (ACLD) are limited.
Methods
Liver disease patients receiving DOAC treatment (ACLD: n = 104; vascular liver disease: n = 29) or vitamin K antagonists (VKA)/low‐molecular‐weight heparin (LMWH; ACLD: n = 45; vascular: n = 13) between January 2010 and September 2020 were retrospectively included. Invasive procedures and bleeding events were recorded. Calibrated anti‐Xa peak levels and thrombomodulin‐modified thrombin generation assays (TM‐TGAs) were measured in a subgroup of 35/28 DOAC patients.
Results
Among patients receiving DOAC, 55 (41.3%) had advanced liver dysfunction (Child‐Pugh‐stage [CPS] B/C) and 66 (49.6%) had experienced decompensation. Overall, 205 procedures were performed in 60 patients and procedure‐related bleedings occurred in 7 (11.7%) patients. Additionally, 38 (28.6%) patients experienced spontaneous (15 minor, 23 major) bleedings during a median follow‐up of 10.5 (IQR: 4.0‐27.8) months. Spontaneous bleedings in ACLD patients were more common in CPS‐B/C (at 12 months: 36.9% vs CPS‐A: 15.9%, subdistribution hazard ratio [SHR]: 3.23 [95% CI: 1.59‐6.58], P < .001), as were major bleedings (at 12 months: 22.0% vs 5.0%, SHR: 5.82 [95% CI: 2.00‐16.90], P < .001). Importantly, CPS (adjusted SHR: 4.12 [91% CI: 1.82‐9.37], P < .001), but not the presence of hepatocellular carcinoma or varices, was independently associated with major bleeding during DOAC treatment. Additionally, ACLD patients experiencing bleeding had worse overall survival (at 12 months: 88.9% vs 95.0% without bleeding; P < .001). Edoxaban anti‐Xa peak levels were higher in patients with CPS‐B/C (345 [95% CI: 169‐395] vs CPS‐A: 137 [95% CI: 96‐248] ng/mL, P = .048) and were associated with lower TM‐TGA. Importantly, spontaneous bleeding rates were comparable to VKA/LMWH patients.
Conclusions
Anticoagulants including DOACs should be used with caution in patients with advanced liver disease due to a significant rate of spontaneous bleeding events.
Keywords: ACLD, bleeding, DOAC, edoxaban, NOAC, vascular liver disease
Abbreviations
- ACLD
advanced chronic liver disease
- BCLC
Barcelona Clinic Liver Cancer
- BCS
Budd‐Chiari syndrome
- CI
confidence interval
- CLD
chronic liver disease
- CSPH
clinically significant portal hypertension
- CPS
Child‐Pugh stage
- DOAC
direct oral anticoagulants
- EBL
endoscopic band ligation
- ERCP
endoscopic retrograde cholangiopancreaticography
- HCC
hepatocellular carcinoma
- LMWH
low‐molecular‐weight heparin
- LVP
large volume paracentesis
- MELD
model for end‐stage liver disease
- PHG
portal hypertensive gastropathy
- PSVD
porto‐sinusoidal vascular disease
- PVT
portal vein thrombosis
- SHR
subdistribution hazard ratio
- VKA
vitamin K antagonist
Key points.
While direct oral anticoagulants (DOACs) are increasingly used in patients with liver disease, safety data especially in advanced chronic liver disease (ACLD) are limited.
Any spontaneous bleeding occurred in 38 patients (28.6%) during treatment with DOAC, of which 23 were graded as major (17.3%).
Incidence of spontaneous bleeding in ACLD patients was significantly higher in CPS‐B/C patients when compared with patients with CPS‐A cirrhosis, and CPS, but not the presence of HCC or varices, was independently associated with major bleeding.
Peak edoxaban anti‐Xa levels were higher and thrombomodulin‐modified thrombin generation assays were lower in CPS‐B/C patients when compared with CPS‐A patients.
1. INTRODUCTION
Patients with advanced chronic liver disease (ACLD) often present with profound changes of coagulation parameters.1 While these patients were traditionally considered to be ‘auto‐anticoagulated’, recent research has shown that even patients with advanced liver disease have a rebalanced equilibrium of pro‐ and anti‐haemostatic factors.2 However, when compared with liver‐healthy subjects, this equilibrium is much more instable and easily tilts towards bleeding or thrombosis. In line, the risk of thrombotic events was shown to be 1.7‐fold increase in patients with cirrhosis compared with the general population,3 and intrasinusoidal microthrombosis followed by parenchymal extinction was even proposed as a driver for disease progression.4 However, these patients are also exposed to an increased risk of bleeding due to clinically significant portal hypertension (CSPH)5 and unstable haemostatic balance.6
A substantial number of patients with ACLD or vascular liver diseases require anticoagulant therapy for the prevention/treatment of venous thromboembolism,7 portal vein thrombosis (PVT),8, 9 or Budd‐Chiari syndrome (BCS). While current guidelines recommend the use of low‐molecular‐weight heparin (LMWH) and vitamin K antagonists (VKA) in this patient population,10 both options have substantial drawbacks.11 LMWH and VKA are rather unpopular treatments as they have to be injected subcutaneously or monitored by INR, which is commonly prolonged in patients with ACLD. Moreover, treatment with VKA interferes with the MELD score potentially masking a worsening of liver function and making timing for liver transplantation listing more difficult. In contrast, direct oral anticoagulants (DOACs) are an interesting alternative and may have practical benefits12: They do not require routine monitoring via INR, do not interfere with MELD score and are taken orally.13 Unfortunately, patients with advanced liver disease were excluded from large randomized DOAC trials.14, 15, 16 Therefore, available evidence largely derives from small retrospective studies suggesting that DOACs are a safe treatment option in patients with compensated ACLD.17 However, there is only very limited data on the safety of DOAC treatment in patients with decompensated liver disease.18 Additionally, these patients frequently need to undergo procedures such as endoscopic band ligation (EBL) and large‐volume paracentesis (LVP),19 and safety of DOAC treatment in this context has yet to be studied.
Even though routine monitoring of DOAC treatment is not required, calibrated anti‐Xa levels may be required in special clinical situations including active bleeding, urgent invasive procedures, renal failure, extreme body weights and in patients that clinically appear resistant to therapy. Whether patients with advanced liver disease would benefit from monitoring is unclear. One study has shown similar anti‐Xa levels in patients with mild cirrhosis on edoxaban compared with healthy individuals,20 but whether drug accumulation may occur in patients with more advanced disease is currently unknown.
Therefore, the aim of this study was to evaluate (a) the safety of DOAC treatment in patients with ACLD or vascular liver diseases in general, (b) the safety of DOAC treatment in liver patients undergoing invasive procedures; and (c) to compare calibrated anti‐Xa‐assay levels between patients with different degrees of liver dysfunction.
2. METHODS
2.1. Study design and population
All patients with advanced parenchymal (ACLD) and/or vascular liver disease treated with DOAC (n = 133) at the Department of Gastroenterology and Hepatology, Medical University of Vienna, between 1 January 2010 and 30 September 2020 were included in this study. Patient characteristics and information on spontaneous and procedure‐related bleedings and the time of procedure and/or bleeding were collected from original patient records that include outpatient letters, discharge letters, procedural and radiological reports and all laboratory reports from a broad network of hospitals in Vienna. Patients with insufficient medical records were excluded. To investigate metabolization of DOACs, calibrated anti‐Xa serum levels were measured in 35 prospectively sampled patients (ClinicalTrials.gov Identifier: NCT03541057) receiving either edoxaban or apixaban (as described below). Thrombomodulin‐modified thrombin generation assays (TM‐TGA) were performed in 28 of these patients to further assess coagulation status. Finally, we also included a cohort of patients with ACLD and/or vascular liver disease treated with LMWH/VKA (n = 58).
2.2. Definitions
Procedure‐related bleedings were defined as associated bleeding events within 4 weeks after EBL, LVP, liver biopsy or surgical interventions.
Bleeding events were graded as minor or major bleedings21: Major bleedings were defined as fatal bleedings, symptomatic bleedings in a critical area or organ (i.e., intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome), bleeding with an associated decrease in haemoglobin level of ≥2 g/dL or bleeding leading to transfusion of two or more units of packed red blood cells. All other clinically evident bleedings were considered minor.
Decompensated liver disease was defined as history of or current ascites, hepatic encephalopathy or portal‐hypertensive bleeding. CSPH was defined as history of or current decompensation, presence of varices or portosystemic collaterals, or HVPG ≥ 10 mm Hg. However, due to the low number of patients in the subgroups, we did not differentiate between pre‐hepatic, intra‐hepatic and post‐hepatic CSPH.
2.3. Measurement of peak calibrated anti‐Xa levels
Blood samples for measurement of peak anti‐Xa levels were drawn from a peripheral vein within 2‐4 hours after drug intake and in a steady state after repeated dosing (no missing dose for at least 3 days). Anti‐Xa levels were measured using a one‐step chromogenic assay (STA‐Liquid Anti‐Xa assay, Diagnostica Stago, 92 600 Asnières‐sur‐Seine, France).
2.4. Thrombomodulin‐modified thrombin generation assay
Blood samples for TM‐TGA measurement were drawn from a peripheral vein at the same time as for the measurement of peak anti‐Xa levels, immediately transferred to the lab, centrifuged once at 1972 g for 10 minutes and stored at −20℃. Stored samples were then transferred to the Surgical Research Laboratory, University Medical Center Groningen, the Netherlands, centrifuged again at 10 000 × g for 10 minutes and measured using a fluorometrical method, as previously described.22, 23, 24 Coagulation was activated using commercially available reagents containing recombinant tissue factor and phospholipids in the presence of soluble thrombomodulin (TM). The following parameters were recorded for this study: endogenous thrombin potential (ETP; representing the total enzymatic work performed by thrombin during the time that it was active) as well as peak thrombin levels.
2.5. Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics 25 (SPSS Inc, USA), R 4.0.5 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism 6.0 (GraphPad Software, Inc, La Jolla, CA, USA). Continuous variables were reported as mean ± standard deviation or median (interquartile range), while categorical variables were reported as proportion of patients with/without a certain characteristic. Student's t test was used for group comparisons of normally distributed variables and Mann‐Whitney U test for non‐normally distributed variables, respectively. Group comparisons of categorical variables were performed using either Pearson's Chi‐squared or Fisher's exact test, as applicable.
Landmark analyses were performed to assess survival after bleeding in order to correct for ‘immortal time bias’.25 In detail, spontaneous bleeding within 6 months or 12 months after DOAC initiation were considered as landmarks. Competing risk analysis was used to compare the incidence of spontaneous bleeding between patients with different severity of liver disease, with or without HCC as well as those receiving full versus reduced DOAC dose, considering death and orthotopic liver transplantation as a competing risk. Therefore, subdistribution hazard ratio (SHR) using the R package ‘cmprsk’ applying Fine and Gray competing risks regression model was used.26 Finally, we applied a multivariable competing risks regression model to identify factors independently associated with spontaneous bleeding. A P‐value ≤ .05 was considered statistically significant.
2.6. Ethics
This study was approved by the ethics committee of the Medical University of Vienna (No. 1928/2017 and 1996/2020). All prospectively included patients (ClinicalTrials.gov identifier: NCT03541057) provided written informed consent. For the retrospective part of this study, the need for written informed consent was waived by the ethics committee.
3. RESULTS
3.1. Baseline characteristics
In total, 133 patients on DOAC with a mean age of 58.1 ± 14.1 years were included. Majority of patients was male (n = 79, 59.4%). While in 104 patients (78.2%), a chronic parenchymal liver disease (ACLD) was diagnosed, 29 patients (21.8%) had vascular liver disease without evidence for ACLD including patients with PSVD (n = 10), PVT without evidence of ACLD (n = 18) and veno‐occlusive disease (n = 1). The most prevalent aetiologies in ACLD patients were alcoholic (n = 25, 24.0%) and non‐alcoholic fatty liver disease (n = 25, 24.0%).
Overall, 66 patients (49.6%) had a history of hepatic decompensation, and 85 patients (63.9%) had varices. Of note, 33 ACLD patients (31.7%) had previously been diagnosed with hepatocellular carcinoma (HCC, BCLC stages at the time of DOAC treatment initiation were: BCLC 0/A: 9.6% BCLC B: 8.7%, BCLC C/D: 13.5%) of whom 16 patients (48.5% of HCC patients) were taking tyrosine kinase inhibitors.
When comparing patients with ACLD and vascular liver disease at baseline, ACLD patients were older (ACLD: 59.5 ± 13.3 vs vascular: 53.0 ± 15.7 years; P = .027), had a worse liver function (Child‐Pugh‐stage [CPS] B/C: ACLD: 51 [49.0%] vs vascular: 4 [13.7%], P = .003) and a higher UNOS MELD‐score (11.7 ± 4.2 vs 8.9 ± 2.7, P < .001). More detailed patient characteristics are displayed in Table 1.
TABLE 1.
Comparison of baseline characteristics between ACLD patients and those with vascular liver disease only
| Characteristics | Overall, n = 133 | ACLD, n = 104 | Vascular liver disease, n = 29a | P value |
|---|---|---|---|---|
| Age, y, mean ± SD | 58.1 ± 14.1 | 59.5 ± 13.3 | 53.0 ± 15.7 | .027 |
| Male sex, n (%) | 79 (59.4%) | 59 (56.7%) | 20 (69.0%) | .235 |
| BMI, kg/m2, mean ± SD | 27.0 ± 5.3 | 27.5 ± 5.4 | 25.1 ± 4.4 | .033 |
| Platelets, G/L | 123 (91‐193) | 116 (87‐176) | 217 (129‐296) | <.001 |
| Albumin, g/dL | 37.9 ± 6.0 | 36.9 ± 6.0 | 40.8 ± 5.1 | .002 |
| Etiology of liver disease, n (%) | ||||
| ALD | 25 (18.8%) | 25 (24.0%) | — | — |
| NAFLD | 25 (18.8%) | 25 (24.0%) | — | |
| Viral hepatitis | 13 (9.8%) | 13 (12.5%) | — | |
| Others | 33 (24.8%) | 32 (30.7%) | 1 (3.4%) | |
| BCS | 9 (6.8%) | 9 (8.7%) | — | |
| PSVD | 10 (7.5%) | — | 10 (34.5%) | |
| PVT without evidence of ACLD | 18 (13.5%) | — | 18 (62.1%) | |
| Clinical characterization of liver disease | ||||
| HCC | 33 (24.8%) | 33 (31.7%) | 0 (0.0%) | <.001 |
| BCLC 0/A | 10 (7.5%) | 10 (9.6%) | 0 (0.0%) | .007 |
| BCLC B | 9 (6.8%) | 9 (8.7%) | 0 (0.0%) | |
| BCLC C/D | 14 (10.5%) | 14 (13.5%) | 0 (0.0%) | |
| Malignancy other than HCC | 11 (8.3%) | 6 (5.8%) | 5 (17.2%) | .061 |
| Decompensated liver disease | 66 (49.6%) | 55 (52.9%) | 11 (37.9%) | .154 |
| Ascites | 57 (42.9%) | 47 (45.2%) | 10 (34.5%) | .303 |
| Hepatic encephalopathy | 22 (16.5%) | 20 (19.2%) | 2 (6.9%) | .159 |
| Varices | 85 (63.9%) | 70 (67.3%) | 15 (51.7%) | .122 |
| Small | 58 (43.6%) | 52 (50.0%) | 6 (20.7%) | .017 |
| Large | 27 (20.3%) | 18 (17.3%) | 9 (31.0%) | |
| PVT | 102 (76.7%) | 77 (74.0%) | 25 (86.2%) | .171 |
| History of variceal bleeding | 14 (10.5%) | 13 (12.5%) | 1 (3.4%) | .302 |
| Portal hypertensive gastropathy | 41 (30.8%) | 37 (35.6%) | 4 (13.8%) | .068 |
| Splenomegaly | 87 (65.4%) | 71 (68.3%) | 16 (55.2%) | .190 |
| History of TIPS | 9 (6.8%) | 7 (6.7%) | 2 (6.9%) | 1.000 |
| Severity of liver disease | ||||
| Child‐Pugh Score, points | 6 (5‐8) | 6 (5‐8) | 5 (5‐6) | .001 |
| A | 78 (58.6%) | 53 (51.0%) | 25 (86.2%) | .003 |
| B | 47 (35.3%) | 44 (42.3%) | 3 (10.3%) | |
| C | 8 (6.0%) | 7 (6.7%) | 1 (3.4%) | |
| UNOS MELD, points | 11.1 ± 4.1 | 11.7 ± 4.2 | 8.9 ± 2.7 | <.001 |
Abbreviations: ALD, alcoholic liver disease; BCLC, Barcelona Clinic Liver Cancer; BCS, Budd‐Chiari syndrome; BMI, body mass index; HCC, hepatocellular carcinoma; MELD, model for end‐stage liver disease; NALFD, non‐alcoholic fatty liver disease; PSVD, porto‐sinusoidal vascular disease; PVT, portal vein thrombosis; SD, standard deviation; TIPS, transjugular intrahepatic portosystemic shunt.
Including patients with PSVD, PVT without parenchymal liver disease and veno‐occlusive disease.
Bold values indicate significance (P ≤ .05)
3.2. Characterization of DOAC treatment
The majority of our patients received edoxaban (n = 75, 56.4%), followed by apixaban (n = 24, 18.0%) and rivaroxaban (n = 24, 18.0%), while only 3 (2.4%) patients were taking dabigatran and 7 patients (5.3%) received more than one drug sequentially. Main indications for DOAC were PVT in 89 patients (66.9%), followed by atrial fibrillation (n = 16, 12.0%) and BCS (n = 9, 6.8%). Seventy‐eight patients (58.6%) received full dose of DOAC (i.e., 60 mg edoxaban once daily, 5 mg apixaban twice daily, 20 mg rivaroxaban once daily or 150 mg dabigatran twice daily), while 55 patients (41.4%) received a reduced dose. Median treatment duration was 10.5 (4.0‐27.8) months and treatment with DOAC was stopped in 38 patients (28.6%). The most common reasons for treatment discontinuation were bleeding events (n = 14, 10.5%), resolution of PVT (n = 8, 6.0%) and decision of the treating physician (n = 5, 3.8%). A detailed comparison between patients with ACLD and vascular liver disease can be found in Table 2.
TABLE 2.
DOAC therapy and bleeding events compared between ACLD patients and those with vascular liver disease only
| Characteristics | Overall, n = 133 | ACLD, n = 104 | Vascular liver disease, n = 29a | P value |
|---|---|---|---|---|
| DOAC type | ||||
| Edoxaban | 75 (56.4%) | 59 (56.7%) | 16 (55.2%) | .468 |
| Apixaban | 24 (18.0%) | 16 (15.4%) | 8 (27.6%) | |
| Rivaroxaban | 24 (18.0%) | 21 (20.2%) | 3 (10.3%) | |
| Dabigatran | 3 (2.4%) | 2 (1.9%) | 1 (3.4%) | |
| Sequential treatment | 7 (5.3%) | 6 (5.8%) | 1 (3.4%) | |
| Indication for anticoagulation | ||||
| PVT | 89 (66.9%) | 65 (62.5%) | 24 (82.8%) | .231 |
| BCS | 9 (6.8%) | 9 (8.7%) | — | |
| Atrial fibrillation | 16 (12.0%) | 15 (14.4%) | 1 (3.4%) | |
| Atrial fibrillation and PVT | 8 (6.0%) | 7 (6.7%) | 1 (3.4%) | |
| DVT ± PE | 8 (6.0%) | 6 (5.8%) | 2 (6.9%) | |
| Others | 3 (2.3%) | 2 (1.9%) | 1 (3.4%) | |
| DOAC dose | ||||
| Full dose | 78 (58.6%) | 61 (58.7%) | 17 (58.6%) | .997 |
| Reduced dose | 55 (41.4%) | 43 (41.3%) | 12 (41.4%) | |
| Duration of DOAC therapy, months | 10.5 (4.0‐27.8) | 10.5 (3.8‐24.8) | 9.0 (4.4‐39.1) | .760 |
| Discontinuation of DOAC therapy | 38 (28.6%) | 34 (32.7%) | 4 (13.8%) | .046 |
| Bleeding | 14 (10.5%) | 11 (10.6%) | 3 (10.3%) | .720 |
| Resolution of PVT | 8 (6.0%) | 7 (6.7%) | 1 (3.4%) | |
| Decision of the treating physician | 5 (3.8%) | 5 (4.8%) | — | |
| Worsening of liver function | 3 (2.3%) | 3 (2.9%) | — | |
| Patient wish | 3 (2.3%) | 3 (2.9%) | — | |
| Others | 5 (3.8%) | 5 (4.8%) | — | |
| Patients with invasive procedures during DOAC therapy | 60 (45.1%) | 47 (45.2%) | 13 (44.8%) | .972 |
| ≥2 interventions | 38 (28.6%) | 31 (29.8%) | 7 (24.1%) | — |
| Number of procedures (overall) | 205 | 177 | 28 | — |
| LVP | 14 (10.5%) | 13 (12.5%) | 1 (3.4%) | .302 |
| Number of LVP (overall) | 87 | 83 | 4 | — |
| EBL | 28 (21.1%) | 20 (19.2%) | 8 (27.6%) | .329 |
| Number of EBL (overall) | 70 | 49 | 21 | — |
| Complications during DOAC therapy | ||||
| Procedure‐related bleedings | 7 (11.7%, 3.4% of all procedures) | 6 (12.8%, 3.4% of all procedures) | 1 (7.7%, 3.6% of all procedures) | 1.000 |
| Minor | 6 | 5 | 1 | — |
| Major | 1 | 1 | 0 | |
| Spontaneous bleeding event | 38 (28.6%) | 33 (31.7%) | 5 (17.2%) | .127 |
| ≥2 spontaneous bleeding events | 4 (3.0%) | 3 (2.9%) | 1 (3.4%) | — |
| Minor | 15 (39.5%, 11.3% of all) | 15 (45.5%, 14.4% of all) | — | .136 |
| Major | 23 (60.5%, 17.3% of all) | 18 (54.5%, 17.3% of all) | 5 (100.0%, 17.2% of all) | |
| Fatal | 1 (2.6%) | 1 (3.0%) | — | — |
Abbreviations: BCS, Budd‐Chiari syndrome; DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; EBL, endoscopic band ligation; LVP, large volume paracentesis; PE, pulmonary embolism; PVT, portal vein thrombosis.
Including patients with PSVD, PVT without parenchymal liver disease and veno‐occlusive disease.
Bold values indicate significance (P ≤ .05)
3.3. Procedure related bleeding events
Almost half of patients (n = 60, 45.1%) underwent at least one intervention during DOAC therapy resulting in a total of 205 invasive procedures during the study period. Specifically, 14 patients (10.5%) underwent LVP resulting in a total of 87 LVPs, and 28 patients (21.1%) underwent EBL resulting in a total of 70 EBLs. DOAC treatment was paused prior to all EBLs as per the discretion of the treating physician. Bleedings during gastroduodenoscopy occurred in 4 patients (14.3%; 5.7% of all EBLs, 3 ACLD patients, 1 patient with vascular liver disease). Apart from these immediate bleedings, no procedure‐related bleeding occurred within 28 days after EBL. Additionally, one minor bleeding occurred after LVP, one minor bleeding occurred during a transurethral resection of the bladder and one major bleeding after endoscopic retrograde cholangiopancreaticography (ERCP). Procedure‐related bleeding events are described in detail in the Supplementary material. A detailed comparison between patients with ACLD and vascular liver disease can be found in Table 2.
3.4. Spontaneous bleeding events during DOAC treatment
Thirty‐eight patients (28.6%) experienced a spontaneous bleeding event during DOAC treatment, while in 4 patients (3.0%) ≥2 events were documented. These events were graded as minor in 15 patients (39.5%) and major in 23 patients (60.5%) of which one event (bleeding from Mallory‐Weiss tear) was followed by acute‐on‐chronic‐liver‐failure and death 9 days after the bleeding event (Table S1). This event occurred in a patient with decompensated CPS‐C cirrhosis at baseline. The majority of spontaneous major bleeding events originated from the gastrointestinal tract. The major non‐gastrointestinal bleedings were due to spontaneous HCC rupture, vaginal bleeding and subdural haematoma. Notably, liver function significantly declined in patients with spontaneous bleeding events between the start of DOAC treatment and the time of bleeding (Child‐Pugh‐score: BL: 7 ± 2 vs bleeding: 8 ± 2, P < .001; MELD: BL: 12.2 ± 4.0 vs bleeding: 15.4 ± 9.2, P = .021). Importantly, while spontaneous bleeding occurred in 31.7% (33/104) of patients with ACLD, it was documented in 17.2% (5/29) of patients with vascular liver disease (P = .127, Table 2).
3.5. Incidence of predisposing factors for spontaneous bleeding events
Next, we compared the cumulative incidence of spontaneous bleeding events between patients with different severity of liver disease. Since risk factors for bleeding may likely differ between ACLD patients and those with vascular liver disease, we focused on ACLD patients for the following analyses.
Interestingly, cumulative incidence of spontaneous bleeding after initiation of DOAC treatment was significantly higher in CPS‐B/C patients when compared with patients with CPS‐A cirrhosis after 12 months (B/C: 36.9% vs A: 15.9%, SHR: 3.23 [95% CI: 1.59‐6.58], P = .001, Figure 1A). These differences were also observed when only considering major bleedings (at 12 months: 22.0% vs 5.0%, SHR: 5.82 [95% CI: 2.00‐16.90], P = .001, Figure 1B). Similar results were obtained in the overall cohort (Figure S1). While a higher incidence of spontaneous bleeding and spontaneous major bleeding could be confirmed in ACLD patients with a history of hepatic decompensation (Figure S2), there was no statistically significant difference with regard to the presence of HCC at baseline or the DOAC dosage (Figures S3 and S4).
FIGURE 1.
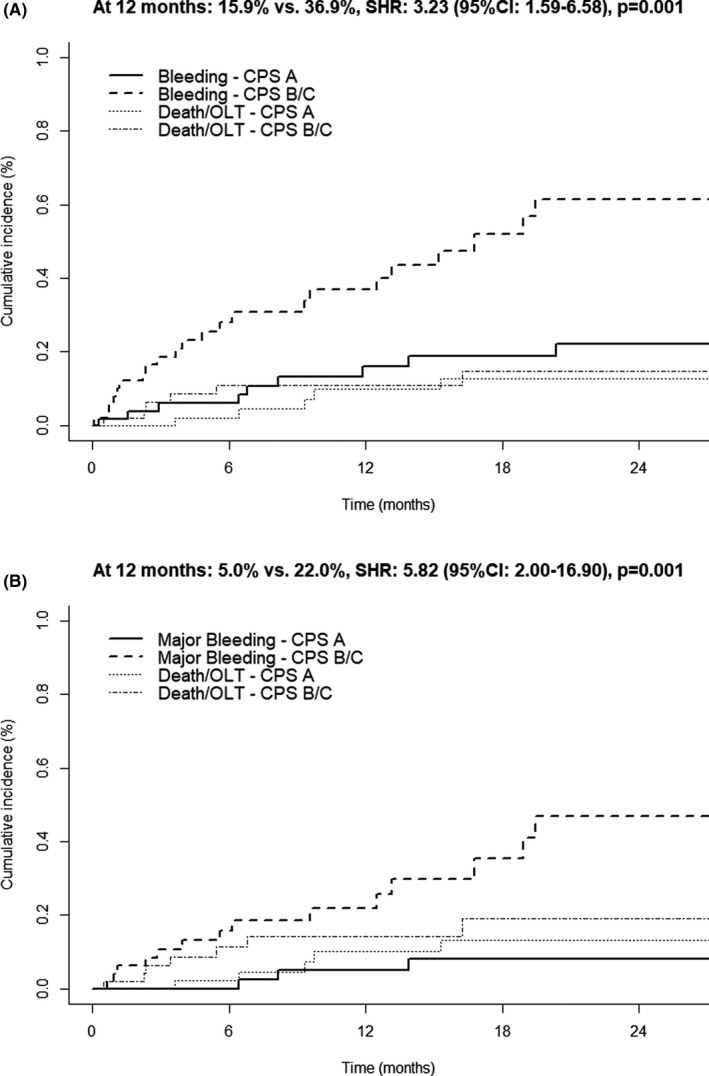
Competing risk analysis comparing the incidence of (A) spontaneous bleeding and (B) spontaneous major bleeding in ACLD patients among Child‐Pugh stage (CPS) A vs CPS B/C considering death and liver transplantation (OLT) as competing risks
Finally, we performed multivariable competing risks regression analyses including CPS, HCC and the presence of varices to identify factors independently associated with the development of spontaneous and spontaneous major bleeding events. Interestingly, while the presence of varices or HCC was not associated with the incidence of bleeding, CPS‐B/C (spontaneous bleedings: adjusted SHR aSHR: 3.30 [91% CI: 1.74‐6.26], P < .001; spontaneous major bleedings: aSHR: 4.12 [91% CI: 1.82‐9.37], P < .001) was independently associated in ACLD patients (Table S2).
3.6. Long‐term survival after bleeding events
As only one immediately fatal bleeding complication occurred, we further investigated whether bleedings during DOAC therapy were associated with worse survival. Therefore, we performed landmark‐analyses and compared the overall survival of ACLD patients who experienced bleeding within 6 months from DOAC initiation to those without bleeding within 6 months. Importantly, bleeding within 6 months was associated with a worse overall survival (at 12 months: 88.9% vs 95.0%, log‐rank P < .001; Figure 2A). The same result was obtained when setting the landmark at 12 months after DOAC initiation (survival at 18 months: 74.1% vs 95.6%, log‐rank P = .011, Figure 2B) as well as in the overall cohort (Figure S5).
FIGURE 2.
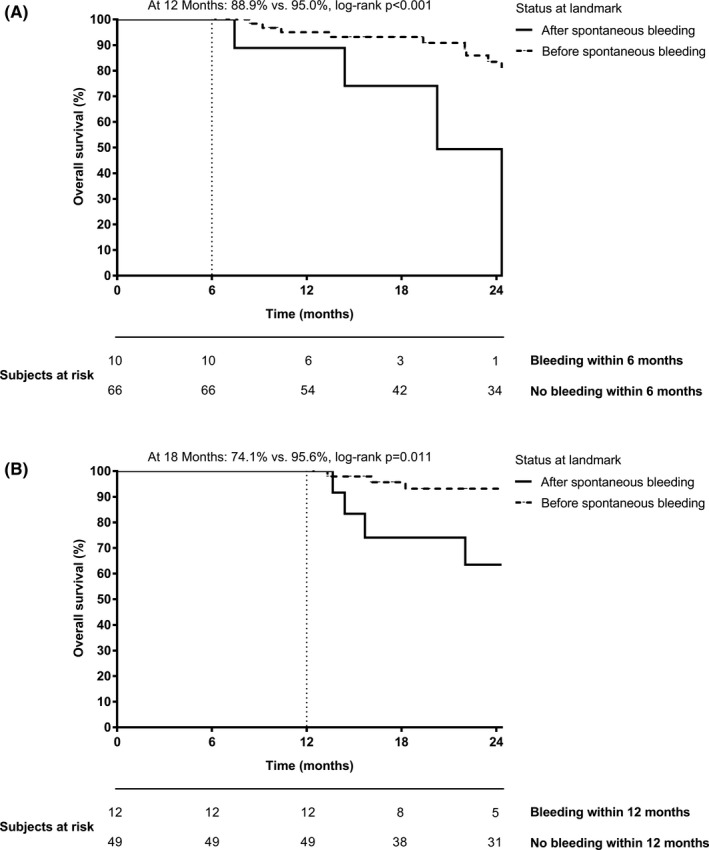
Survival after bleeding events using landmark analysis comparing the survival of ACLD patients with and without bleeding (A) within 6 months and (B) within 12 months. Importantly, patients were only considered if they were followed for at least (A) 6 months or (B) 12 months
3.7. Calibrated anti‐Xa peak levels
In order to study changes in pharmacokinetics potentially explaining the substantial risk of spontaneous bleeding during DOAC treatment, we measured calibrated anti‐Xa levels in 35 prospectively sampled patients. As shown in Table S3, patients with anti‐Xa measurement had less advanced liver disease compared with patients not undergoing anti‐Xa measurements. Consecutively, overall bleeding incidence was lower in the anti‐Xa cohort which is in line with our findings outlined above (Figure S6). In total, anti‐Xa peak levels were measured in 23 patients with edoxaban and in 12 patients with apixaban treatment. As shown in Figure 3, peak edoxaban anti‐Xa levels were numerically higher in CPS‐B/C patients, when compared with CPS‐A patients (226 [70‐350] vs 137 [86‐199] ng/mL, P = .301), and this difference attained statistical significance when only including patients who were dosed according to the label (345 [169‐395] vs 137 [96‐248] ng/mL, P = .048) as 3 patients in the CPS‐B/C group and 4 patients in the CPS‐A did not receive the recommended drug dose. Unfortunately, the number of patients receiving apixaban was significantly lower which also resulted in a lower number of anti‐Xa measurements (n = 12). However, median anti‐Xa levels tended to be higher in patients with CPS‐B/C vs CPS‐A cirrhosis (297 [162‐356] vs 138 [80‐189] ng/mL, P = .073).
FIGURE 3.
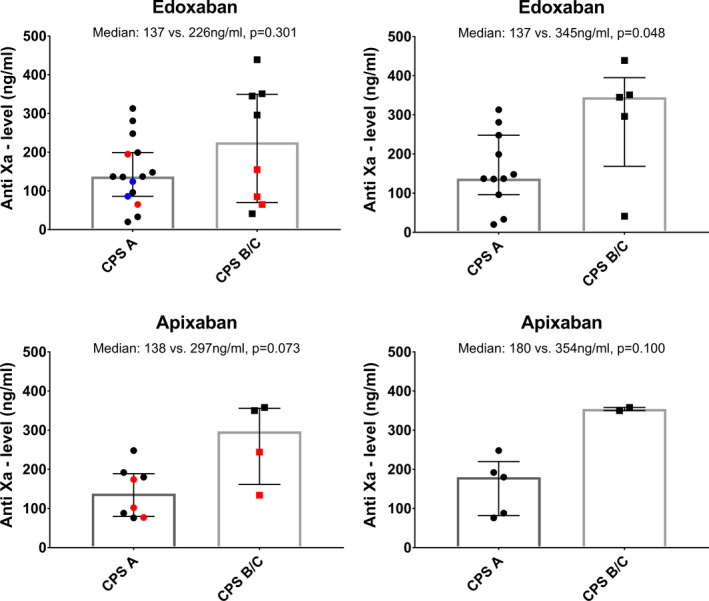
Comparison of calibrated anti‐Xa peak levels in patients with edoxaban and apixaban treatment according to Child‐Pugh stage (left panel). Red dots indicate underdosing while blue dots indicate overdosing as according to the label. The right panels only include patients who were treated according to the dosing recommendations of the respective drug
3.8. Thrombomodulin‐modified thrombin generation assay
Next, we evaluated the correlation of peak anti‐Xa values with ETP as measured by TM‐TGA in 28 prospectively sampled patients. Interestingly, and as depicted in Figure S7, an exponential relationship was observed for anti‐Xa levels and ETP (R 2 = 0.119, P = .072, y = 351.314 × x^[−0.181]) as well as for peak levels (R 2 = 0.121, P = .070, y = 235.462 × x^[−0.155]). Importantly, while patients with higher anti‐Xa values (≥100 ng/mL) generated low amounts of thrombin (ETP: 83 [34‐605] nM; peak: 18 [5‐170] nM), thrombin generation tended to be higher in patients with low anti‐Xa values (<100 ng/mL: ETP: 466 [281‐941] nM; peak: 106 [52‐239] nM; ETP: P = .051, peak: P = .057) despite small numbers (Table S4).
3.9. Comparison to patients on LMWH/VKA
Finally, we compared the bleeding incidence in DOAC patients to patients receiving LMWH or VKA (n = 58). As shown in Table S5, baseline characteristics of the two cohorts were comparable. However, treatment indications were significantly different. While DOAC patients had more often portal vein thrombosis, atrial fibrillation was more common in patients with LMWH/VKA treatment (Table S5). Incidence of procedure‐related bleedings (DOAC: 11.7% vs LMWH/VKA: 12.8%, P = .162) was comparable between the two cohorts. Importantly, spontaneous bleedings were numerically more common in DOAC patients (28.6% vs 19.0%, P = .162). Additionally, the results of multivariable competing risks regression models in the whole cohort (including DOAC and LMWH/VKA patients) were similar to the analyses obtained in the DOAC cohort only. Again, CPS‐B/C was the only factor independently associated with major bleedings, whereas the presence of varices, HCC or DOAC intake was no risk factor (Table S6).
4. DISCUSSION
DOACs are the treatment of choice for non‐valvular atrial fibrillation and thromboembolism due to a more favourable safety profile compared with VKAs as well as the easier application compared with LMWH. Therefore, DOACs are commonly used in subjects with preserved liver and kidney function; however, their use remains off‐label in patients with ACLD, as patients with ‘clinically significant liver disease’ (apixaban),14 ‘significant liver disease’ (rivaroxaban)15 or ‘active liver disease’ (edoxaban)16 have been excluded from clinical trials. In line with the respective package inserts, these drugs are contraindicated in ACLD patients with coagulopathy and clinically relevant risk for bleeding complications.27 Nevertheless, these drugs are also increasingly used in clinical practice in patients with advanced liver disease.
While DOACs seem to be safe in patients with compensated ACLD, data on patients with decompensated ACLD are scarce.17, 28, 29 While bleeding rates reported by some small retrospective studies17, 30 were even lower when compared with historic controls treated with VKA or LMWH,12, 31 a recent study reported a substantial bleeding risk in patients with decompensated cirrhosis. In this study by Mort et al,18 21% of patients with decompensated cirrhosis stopped DOAC treatment due to actual or ‘perceived’ bleeding. In total, bleeding events occurred in every third patient in our as well as their study. However, the rate of major bleeding tended to be higher in our study (17.3% vs 8.0% in the US study). Of note, the median time of DOAC exposure was only 6.0 months in the latter study compared with 10.5 months in our cohort. The differences in bleeding rates may also be explained by the drugs used. While apixaban, a drug taken twice daily, was commonly prescribed in the US study (68.1% of patients), edoxaban was chosen in the majority of our patients (56.4%). Differences in major bleedings may also be explained by different indications for DOAC treatment in the two studies. While PVT was the indication for anticoagulation in 28.3% of patients in the study by Mort et al,18 PVT was present in 76.7% of patients in our study. It has been shown that PVT may cause portal‐hypertensive bleedings that are more difficult to treat when compared with non‐PVT portal hypertensive bleedings32, 33; however, the presence of varices was not associated with the incidence of bleeding in our cohort highlighting the importance of a proper portal hypertension management.34
Importantly, we observed that CPS‐B/C patients receiving DOAC had a significantly higher risk for spontaneous bleeding when compared with patients with CPS‐A cirrhosis. While bleeding episodes in this population may be related to a DOAC‐associated haemostatic failure, it cannot be excluded that these bleedings were triggered by other risk factors such as portal hypertension19 or tyrosine kinase inhibitor therapy.35
While no baseline or laboratory parameter was predictive of spontaneous bleeding in the US study,18 we did observe an association of spontaneous bleeding with liver function. However, it is unclear whether the observed increased bleeding risk simply reflects the more fragile haemostatic balance in patients with more pronounced liver dysfunction or is caused by DOAC treatment.
Importantly, spontaneous bleeding events were numerically, but not statistically significantly more common in DOAC patients compared with LMWH/VKA patients. Although this may suggest a higher bleeding risk in patients with advanced liver disease receiving DOAC, these results may be biased in several ways: First, PVT was more common in DOAC patients, and previous studies have demonstrated that PVT is associated with a higher bleeding risk compared with patients being anticoagulated for other indications.7 Furthermore, we believe that the decision of the treating physicians to recommend DOAC over LMWH/VKA treatment was for good – but non‐documented – reasons that rendered patients as appropriate candidates for DOAC therapy. Finally, the easier mode of treatment is of particular relevance in some patients, such as suggested by the significantly higher number of patients with hepatic encephalopathy in the DOAC cohort. Still, the retrospective comparison of the two treatment strategies has several drawbacks and prospective randomized trials comparing DOAC and LMWH/VKA treatment in patients with advanced liver dysfunction are urgently needed.
Until results from such trials will be available, use of DOACs in patients with advanced liver disease is challenging as it is unknown whether portal hypertensive gastropathy/enteropathy influences drug uptake as well as possible effects of liver dysfunction on drug metabolism.36 As DOACs are cleared by the liver and the kidney to a different extent, drug accumulation with a possibly increased risk for bleeding is a major concern. Interestingly, recent data published by Bos et al20 suggested that even though edoxaban drug levels after 7 days of treatment were comparable to those in liver‐healthy subjects, the anticoagulatory potential of edoxaban might be weaker in liver disease patients. However, this study mostly included CPS‐A and did not include a sufficient number of patients with more advanced liver disease and thus does not provide information on patients with advanced liver dysfunction and portal hypertension, that is, those who at the same time show the most pronounced procoagulant imbalance and are at the highest risk of bleeding.
In our study, we found that peak anti‐Xa levels were significantly higher in patients with more advanced liver disease, potentially explaining the increased bleeding incidence in this subgroup. In line, median peak levels in our cohort of CPS‐B/C patients were considerably higher than the values reported in the general population.37, 38 Additionally, when performing TM‐TGA assays, thrombin generation was lower in patients with higher anti‐Xa levels when compared with patients with low anti‐Xa levels indicating that high anti‐Xa levels may reflect an impaired haemostasis.39 Importantly, peak anti‐Xa levels and TM‐TGAs were measured in a steady state of DOAC treatment after repeated intake, which may be most reflective of the anticoagulation‐related bleeding risk during long‐term treatment. This may also explain the different results obtained in single dosing studies reporting reduced plasma concentrations in patients with advanced liver disease.40
Apart from spontaneous bleeding events, the rate of post‐procedural bleeding needs to be taken into account when considering DOAC treatment in a patient with decompensated cirrhosis. A recent study showed that the rate of bleeding after variceal ligation was not increased in patients receiving LMWH (3.8% vs 1.6%),41 while this rate was higher in another study (9%).42 However, the rate of post‐procedural bleeding has not been studied in patients receiving DOAC treatment yet. In our study, we observed a low rate of procedure‐related bleedings (11.7% of all patients undergoing any procedure, 3.4% of all procedures), although almost half of patients (45.1%) underwent at least one procedure resulting in a total of 205 procedures. Additionally, rules for pausing DOACs prior to an intervention currently only consider renal function and the bleeding risk of the planned intervention and do not control for liver function.43 Nevertheless, we observed an excellent safety profile of DOACs in patients across different stages of liver disease when drugs were paused as recommended, although some intra‐procedural bleedings occurred, which, however, seemed to be unrelated to haemostasis in most cases.
This study has some limitations. Although we had access to the clinical documentation system from a broad network of hospitals, the retrospective design limits the accuracy of detecting minor bleedings since those might not be completely documented. However, we are confident to have included all major and procedure‐related bleedings since they usually require in‐hospital therapy and consequently get followed at our institution. Secondly, we aimed at linking the incidence of bleeding to anti‐Xa levels in the respective patients. However, measurements were only performed in a subgroup of patients since the assays were only recently established, active intake of DOAC at the time of measurement and patient compliance were required and COVID‐19 restrictions made sample collection more difficult. Nevertheless, we are confident that the above‐mentioned results of the pharmacokinetic study may be extrapolated to the whole cohort. Thirdly, the correlation between peak anti‐Xa values and ETP deserves further research since the low sample size and a high interindividual variability of these advanced coagulation tests limit their interpretation. Therefore, these results should be considered hypothesis‐generating, and further research is needed to evaluate the utility of measuring anti‐Xa levels as well as TM‐TGA in ACLD patients with DOAC therapy. Finally, there was no statistically significant difference in the bleeding incidence between patients receiving DOAC and VKA/LMWH. However, this comparison may be biased by indication, as it was left to the discretion of the treating physician to choose from the available anticoagulation options.
In conclusion, we provide comprehensive data on DOAC safety in a large retrospective cohort of patients with ACLD or vascular liver diseases. When drugs are paused as recommended, DOAC therapy is not associated with a significant risk of procedure‐related bleedings among patients with different stages of liver disease. However, we observed a significant association of spontaneous bleedings with liver disease severity. Therefore, DOACs should be used with caution in this patient group, and liver function should be monitored regularly. Finally, anti‐Xa and TM‐TGA measurements could be useful to evaluate the bleeding risk in individual ACLD patients requiring DOAC therapy.
CONFLICTS OF INTEREST
The authors have nothing to disclose regarding the work under consideration for publication. The following authors disclose conflicts of interests outside the submitted work: GS, KP, LB, TBi, LH, JB and TL have nothing to disclose. DB received travel support from AbbVie and Gilead. BSi received travel support from Abbvie and Gilead. PS received speaking honoraria from Bristol‐Myers Squibb and Boehringer‐Ingelheim, consulting fees from PharmaIN and travel support from Falk. TBu received travel support from AbbVie, Bristol‐Myers Squibb and Medis as well as speaker fees from Bristol‐Myers Squibb. MP is an investigator for Bayer, BMS, Lilly and Roche; he received speaker honoraria from Bayer, BMS, Eisai, Lilly and MSD; he is a consultant for Bayer, BMS, Ipsen, Eisai, Lilly, MSD and Roche; he received travel support from Bayer and BMS. MT received grant support from Albireo, Cymabay, Falk, Gilead, Intercept, MSD and Takeda, honoraria for consulting from Albireo, Boehringer Ingelheim, BiomX, Falk, Genfit, Gilead, Intercept, Jannsen, MSD, Novartis, Phenex and Regulus, speaker fees from Bristol‐Myers Squibb, Falk, Gilead, Intercept and MSD as well as travel support from AbbVie, Falk, Gilead and Intercept. MM served as a speaker and/or consultant and/or advisory board member for AbbVie, Bristol‐Myers Squibb, Collective Acumen, Gilead and W. L. Gore & Associates and received travel support from AbbVie, Bristol‐Myers Squibb and Gilead. JS received grant support from Eli Lilly and Company and Gilead. TR received grant support from AbbVie, Boehringer‐Ingelheim, Gilead, MSD, Gore, Philips Healthcare, Pliant Pharmaceuticals, Siemens; speaking honoraria from AbbVie, Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fee from AbbVie, Bayer, Boehringer‐Ingelheim, Gilead, Intercept, MSD, Siemens; and travel support from Boehringer‐Ingelheim, Gilead and Roche. BS received travel support from AbbVie, Ipsen and Gilead.
Supporting information
Supplementary Material
Semmler G, Pomej K, Bauer DJM, et al. Safety of direct oral anticoagulants in patients with advanced liver disease. Liver Int. 2021;41:2159–2170. 10.1111/liv.14992
Handling Editor: Raúl Andrade
Georg Semmler and Katharina Pomej contributed equally to this study.
Funding information
This work was supported by a grant of the Medical‐scientific fund of the Mayor of the federal capital Vienna awarded to BS (BMF 18062).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Intagliata NM, Argo CK, Stine JG, et al. Concepts and controversies in haemostasis and thrombosis associated with liver disease: proceedings of the 7th international coagulation in liver disease conference. Thromb Haemost. 2018;118:1491‐1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878‐885. [DOI] [PubMed] [Google Scholar]
- 3.Ambrosino P, Tarantino L, Di Minno G, et al. The risk of venous thromboembolism in patients with cirrhosis. A systematic review and meta‐analysis. Thromb Haemost. 2017;117:139‐148. [DOI] [PubMed] [Google Scholar]
- 4.Wanless IR, Wong F, Blendis LM, et al. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238‐1247. [PubMed] [Google Scholar]
- 5.Reiberger T, Ulbrich G, Ferlitsch A, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non‐response to propranolol. Gut. 2013;62:1634‐1641. [DOI] [PubMed] [Google Scholar]
- 6.Lisman T, Violi F. Cirrhosis as a risk factor for venous thrombosis. Thromb Haemost. 2017;117:3‐5. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi F, Maria N, Villa E. Anticoagulation in cirrhosis: a new paradigm? Clin Mol Hepatol. 2017;23:13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez‐Gea V, De Gottardi A, Leebeek FWG, et al. Current knowledge in pathophysiology and management of Budd‐Chiari syndrome and non‐cirrhotic non‐tumoral splanchnic vein thrombosis. J Hepatol. 2019;71:175‐199. [DOI] [PubMed] [Google Scholar]
- 9.Plessier A, Darwish‐Murad S, Hernandez‐Guerra M, et al. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow‐up study. Hepatology. 2010;51:210‐218. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary JG, Greenberg CS, Patton HM, et al. AGA Clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157:34‐43.e1. [DOI] [PubMed] [Google Scholar]
- 11.Tripodi A, Primignani M, Braham S, et al. Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists. Dig Liver Dis. 2016;48:1208‐1213. [DOI] [PubMed] [Google Scholar]
- 12.Lee HF, Chan YH, Chang SH, et al. Effectiveness and safety of non‐vitamin K antagonist oral anticoagulant and warfarin in cirrhotic patients with nonvalvular atrial fibrillation. J Am Heart Assoc. 2019;8:e011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Intagliata NM, Maitland H, Caldwell SH. Direct oral anticoagulants in cirrhosis. Curr Treat Options Gastroenterol. 2016;14:247‐256. [DOI] [PubMed] [Google Scholar]
- 14.Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2012;368:699‐708. [DOI] [PubMed] [Google Scholar]
- 15.The EINSTEIN Investigators . Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499‐2510. [DOI] [PubMed] [Google Scholar]
- 16.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093‐2104. [DOI] [PubMed] [Google Scholar]
- 17.De Gottardi A, Trebicka J, Klinger C, et al. Antithrombotic treatment with direct‐acting oral anticoagulants in patients with splanchnic vein thrombosis and cirrhosis. Liver Int. 2017;37:694‐699. [DOI] [PubMed] [Google Scholar]
- 18.Mort JF, Davis JPE, Mahoro G, et al. Rates of bleeding and discontinuation of direct oral anticoagulants in patients with decompensated cirrhosis. Clin Gastroenterol Hepatol. 2021;19:1436‐1442. [DOI] [PubMed] [Google Scholar]
- 19.Reiberger T, Puspok A, Schoder M, et al. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien Klin Wochenschr. 2017;129:135‐158. 10.1007/s00508-017-1262-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bos S, Schreuder T, Blokzijl H, et al. Anticoagulant activity of edoxaban in patients with cirrhosis. Blood. 2020;136:1561‐1564. [DOI] [PubMed] [Google Scholar]
- 21.Schulman S, Kearon C, Tsocoaot S, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692‐694. [DOI] [PubMed] [Google Scholar]
- 22.Lisman T, Adelmeijer J. Preanalytical variables affect thrombomodulin‐modified thrombin generation in healthy volunteers. Thromb Res. 2020;194:237‐239. [DOI] [PubMed] [Google Scholar]
- 23.Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4‐15. [DOI] [PubMed] [Google Scholar]
- 24.Bos S, van den Boom B , Kamphuisen PW, et al. Haemostatic profiles are similar across all aetiologies of cirrhosis. Thromb Haemost. 2019;119:246‐253. [DOI] [PubMed] [Google Scholar]
- 25.Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time‐dependent events. Transpl Int. 2018;31:125‐130. [DOI] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496‐509. [Google Scholar]
- 27.Kubitza D, Roth A, Becka M, et al. Effect of hepatic impairment on the pharmacokinetics and pharmacodynamics of a single dose of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol. 2013;76:89‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Intagliata NM, Maitland H, Northup PG, et al. Treating thrombosis in cirrhosis patients with new oral agents: ready or not? Hepatology. 2015;61:738‐739. [DOI] [PubMed] [Google Scholar]
- 29.Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61:1721‐1727. [DOI] [PubMed] [Google Scholar]
- 30.Scheiner B, Stammet PR, Pokorny S, et al. Anticoagulation in non‐malignant portal vein thrombosis is safe and improves hepatic function. Wien Klin Wochenschr. 2018;130:446‐455. 10.1007/s00508-018-1351-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoolwerf EW, Kraaijpoel N, Büller HR, et al. Direct oral anticoagulants in patients with liver cirrhosis: a systematic review. Thromb Res. 2018;170:102‐108. [DOI] [PubMed] [Google Scholar]
- 32.D'Amico G, De Franchis R. Upper digestive bleeding in cirrhosis. Post‐therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599‐612. [DOI] [PubMed] [Google Scholar]
- 33.European Association for the Study of the Liver . Electronic address eee. EASL clinical practice guidelines: vascular diseases of the liver. J Hepatol. 2016;64:179‐202. [DOI] [PubMed] [Google Scholar]
- 34.de Franchis R , Baveno VIF. Expanding consensus in portal hypertension: report of the baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743‐752. [DOI] [PubMed] [Google Scholar]
- 35.Pomej K, Scheiner B, Park D, et al. Vascular complications in patients with hepatocellular carcinoma treated with sorafenib. Cancers (Basel). 2020;12:2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elhosseiny S, Al Moussawi H, Chalhoub JM, et al. Direct oral anticoagulants in cirrhotic patients: current evidence and clinical observations. Can J Gastroenterol Hepatol. 2019;2019:4383269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gosselin RC, Adcock DM, Bates SM, et al. International council for standardization in haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118:437‐450. [DOI] [PubMed] [Google Scholar]
- 38.Weitz JI, Connolly SJ, Patel I, et al. Randomised, parallel‐group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost. 2010;104:633‐641. [DOI] [PubMed] [Google Scholar]
- 39.Bosch Y, Al Dieri R, ten Cate H , et al. Preoperative thrombin generation is predictive for the risk of blood loss after cardiac surgery: a research article. J Cardiothorac Surg. 2013;8:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graff J, Harder S. Anticoagulant therapy with the oral direct factor Xa inhibitors rivaroxaban, apixaban and edoxaban and the thrombin inhibitor dabigatran etexilate in patients with hepatic impairment. Clin Pharmacokinet. 2013;52:243‐254. [DOI] [PubMed] [Google Scholar]
- 41.Bianchini M, Cavani G, Bonaccorso A, et al. Low molecular weight heparin does not increase bleeding and mortality post‐endoscopic variceal band ligation in cirrhotic patients. Liver Int. 2018;38:1253‐1262. [DOI] [PubMed] [Google Scholar]
- 42.Ponthus S, Spahr L, Casini A, et al. Safety of variceal band ligation in patients with cirrhosis and portal vein thrombosis treated with anticoagulant therapy: a retrospective study. Eur J Gastroenterol Hepatol. 2020;32:395‐400. [DOI] [PubMed] [Google Scholar]
- 43.Veitch AM, Vanbiervliet G, Gershlick AH, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British society of gastroenterology (BSG) and European society of gastrointestinal endoscopy (ESGE) guidelines. Endoscopy. 2016;48:385‐402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


