Figure 1.
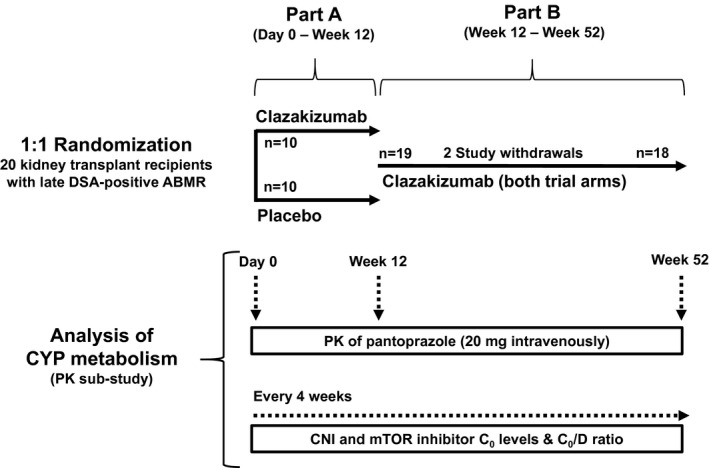
Summary of trial protocol. Twenty renal allograft recipients diagnosed with late antibody‐mediated rejection (ABMR) were randomized to receive clazakizumab or placebo for 12 weeks (part A). After 12 weeks, patients entered part B and they all were scheduled to receive clazakizumab. Two patients were withdrawn from the study because of diverticular disease complications. Both completed part A, one was withdrawn shortly before, the other after the first clazakizumab dose in part B. C0, trough level; CNI, calcineurin inhibitor; CYP, cytochrome P450; D, dose; DSA, donor‐specific antibody; mTOR, mammalian target of rapamycin; PK, pharmacokinetics.
