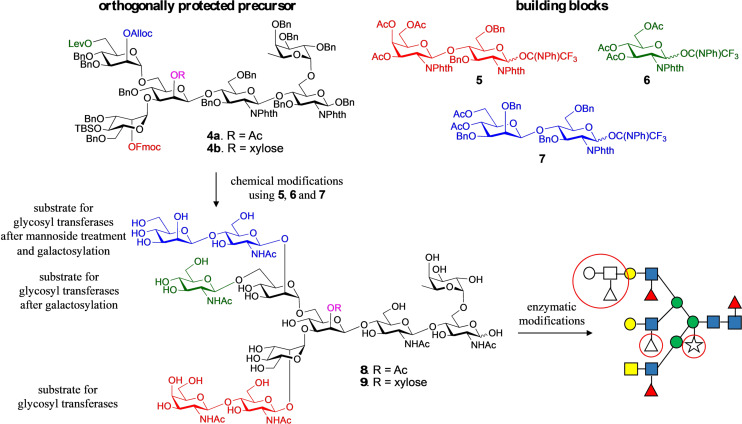
Figure 2.
Overview of the synthetic strategy for the preparation of asymmetrical glycans with and without core xyloside. The coupling of the common precursors, 4 a and 4 b, which display orthogonal protecting groups at key branching points, with glycosyl donors 5–7 followed by global deprotection afforded asymmetric tri‐antennary glycans, 8 and 9, respectively. The latter intermediates were used as substrates for enzymatic extensions of each antenna to yield the asymmetrical glycans 1, 2 and 3, which differ for in presence or absence of core‐xylose, fucosylation of the arms and for the presence of a di‐LeX moiety. Antenna selective arm extension was possible because the unnatural β‐mannoside at the C‐2′ arm temporarily blocks it from enzymatic modification. It can, however, be unmasked by a β‐mannosidase and after enzymatic galactosylation, a precursor is obtained that can be elaborated by many glycosyl transferases into a complex structure. The strategy also exploits that many glycosyl transferases can modify LacNAc but not GlcNAc. The latter can, however, be converted into LacNAc by galactosylation with GalT1. Key structural features are highlighted by red circles.