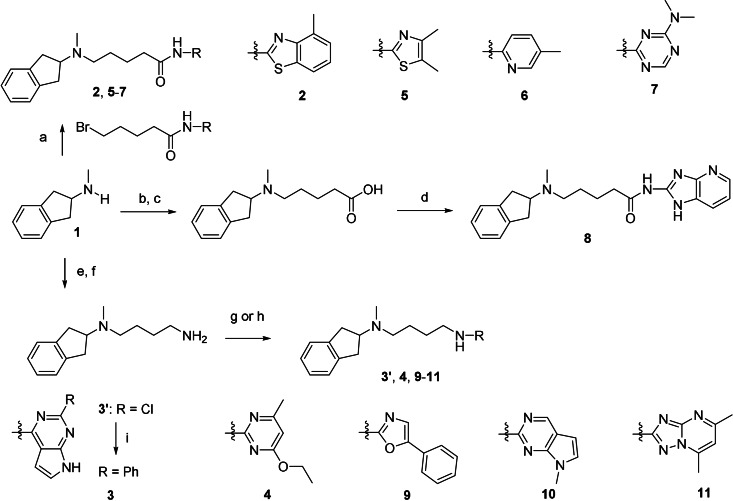
Scheme 1.
Synthesis of compounds 2–11. Reagents and conditions: a) N‐aryl‐5‐bromopentanamides, K2CO3, DMF, RT, overnight, 6–65 % (HPLC); b) ethyl 5‐bromopentanoate, K2CO3, DMF, RT, overnight, 70 %; c) KOH, MeOH, 85 %; d) 1H‐imidazo[4,5‐b]pyridin‐2‐amine, HATU, DIEA, DMF/DCM, RT, overnight, 22 % (HPLC); e) 4‐bromobutanenitrile, K2CO3, CH3CN, RT, overnight, 24 %; f) LiAlH4, Et2O, 1 h, 82 % (HPLC); g) aryl chlorides, K2CO3, CH3CN, 70–160 °C, 1–4 h, 40–51 % (HPLC) for 3′, 4, and 9; h) aryl chloride or bromide, CuI, 1,10‐phenantroline, K2CO3, DMF, 120 °C, 48 h, 2–28 % (HPLC) for 10 and 11; i) phenyl boronic acid, Pd(PPh3)4, K2CO3, dioxane/H2O, 100 °C, overnight, 20 % (HPLC, over 2 steps).