Table 2.
Experimental data for optimized dual‐target ligands.
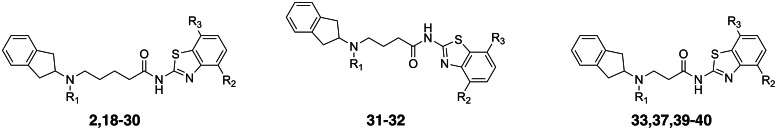
|
Cmpd |
Structure |
Binding affinity[a] |
Functional activity[a] |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
R1 |
R2 |
R3 |
A2AAR |
D2R |
A2AAR |
D2R |
|
|
|
|
|
|
Ki [μm] |
Ki [μm] |
Kb [μm] |
EC50 [μm] |
Emax [%] |
|
2 |
CH3 |
CH3 |
H |
1.2±0.1 |
0.90±0.08 |
–[b] |
9.7±3.8 |
93±12 |
|
18 |
CH3 |
OCH3 |
H |
0.37±0.05 |
0.39±0.03 |
– |
26±14 |
89±18 |
|
19 |
CH3 |
Cl |
H |
0.42±0.04 |
0.51±0.05 |
– |
23±9 |
82±22 |
|
20 |
CH3 |
Br |
H |
0.19±0.03 |
0.34±0.03 |
0.32±0.03 |
1.5±0.02 |
62±9 |
|
21 |
CH2CH3 |
OCH3 |
H |
0.63±0.08 |
0.33±0.02 |
– |
– |
– |
|
22 |
CH2CH3 |
Br |
H |
0.34±0.05 |
0.34±0.04 |
– |
2.5±0.9 |
119±6 |
|
23 |
CH2CH2CH3 |
F |
H |
2.9±0.6 |
0.20±0.02 |
– |
– |
– |
|
24 |
CH2CH2CH3 |
CH2CH3 |
H |
11±3 |
0.82±0.18 |
– |
– |
– |
|
25 |
CH2CH2CH3 |
CH3 |
H |
0.99±0.24 |
0.34±004 |
– |
0.75±0.07 |
81±5 |
|
26 |
CH2CH2CH3 |
OCH3 |
H |
0.61±0.09 |
0.23±0.02 |
3.6±2.0 |
0.99±0.38 |
94±3 |
|
27 |
CH2CH2CH3 |
Br |
H |
0.39±0.05 |
0.53±0.18 |
0.51±0.24 |
0.18±0.03 |
89±4 |
|
28 |
CH2CH2CH3 |
OCH3 |
Cl |
0.47±0.11 |
0.90±0.10 |
– |
1.2±0.2 |
104±6 |
|
29 |
CH2CH2CH3 |
OCH3 |
CH3 |
0.46±0.04 |
0.67±0.07 |
1.9±0.7 |
0.98±0.01 |
105±5 |
|
30 |
CH2CH2CH3 |
OCH3 |
OCH3 |
0.16±0.03 |
0.37±0.03 |
0.72±0.25 |
0.18±0.04 |
77±5 |
|
31 |
CH3 |
CH3 |
H |
1.6±0.3 |
0.63±0.05 |
– |
5.2±2.6 |
88±13 |
|
32 |
CH3 |
Br |
H |
0.67±0.11 |
0.88±0.11 |
– |
1.2±0.3 |
51±4 |
|
33 |
CH3 |
Br |
H |
1.1±0.2 |
2.2±0.3 |
– |
5.0±2.8 |
81±6 |
|
37 |
CH3 |
OCH3 |
OCH3 |
0.30±0.05 |
1.3±0.2 |
– |
31±12 |
103±3 |
|
39 |
CH2CH3 |
OCH3 |
CH3 |
1.3±0.2 |
2.0±0.3 |
– |
28±9 |
107±6 |
|
40 |
CH2CH3 |
OCH3 |
OCH3 |
0.72±0.07 |
1.9±0.3 |
– |
8.9±2.7 |
105±5 |
[a] Data represent mean values±SEM of three individual experiments each performed in duplicate. Emax values are relative (%) to the maximal effect of dopamine. [b] Not determined.
