Abstract
Purpose
To propose and validate a fully automated multicriterial treatment planning solution for a CyberKnife® equipped with an InCiseTM 2 multileaf collimator.
Methods
The AUTO BAO plans are generated using fully automated prioritized multicriterial optimization (AUTO MCO) of pencil‐beam fluence maps with integrated noncoplanar beam angle optimization (BAO), followed by MLC segment generation. Both the AUTO MCO and segmentation algorithms have been developed in‐house. AUTO MCO generates for each patient a single, high‐quality Pareto‐optimal IMRT plan. The segmentation algorithm then accurately mimics the AUTO MCO 3D dose distribution, while considering all candidate beams simultaneously, rather than replicating the fluence maps. Pencil‐beams, segment dose depositions, and final dose calculations are performed with a stand‐alone version of the clinical dose calculation engine. For validation, AUTO BAO plans were generated for 33 prostate SBRT patients and compared to reference plans (REF) that were manually generated with the commercial treatment planning system (TPS), in absence of time pressure. REF plans were also compared to AUTO RB plans, for which fluence map optimization was performed for the beam angle configuration used in the REF plan, and the segmentation could use all these beams or only a subset, depending on the dosimetry.
Results
AUTO BAO plans were clinically acceptable and dosimetrically similar to REF plans, but had on average reduced numbers of beams ((beams in AUTO BAO)/(beams in REF) (relative improvement): 24.7/48.3 (−49%)), segments (59.5/98.9 (−40%)), and delivery times (17.1/22.3 min. (−23%)). Dosimetry of AUTO RB and REF were also similar, but AUTO RB used on average fewer beams (38.0/48.3 (−21%)) and had on average shorter delivery times (18.6/22.3 min. (−17%)). Delivered Monitor Units (MU) were similar for all three planning approaches.
Conclusions
A new, vendor‐independent optimization workflow for fully automated generation of deliverable high‐quality CyberKnife® plans was proposed, including BAO. Compared to manual planning with the commercial TPS, fraction delivery times were reduced by 5.3 min. (−23%) due to large reductions in beam and segment numbers.
Keywords: automated treatment planning, beam angle and beam profile optimization, BAO and IMRT, prostate SBRT, robotic CyberKnife® radiotherapy
1. INTRODUCTION
Quality of clinical treatment plans can vary drastically,1, 2, 3, 4 for example, depending on the skills and ambition of the planner, the complexity of the case, and the time available for planning. Automated treatment planning can be used to improve the quality and consistency of treatment plans (e.g. Ref. [5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17]), and can also substantially reduce the treatment planning workload.
In our center, Erasmus‐iCycle has been developed for automated multicriterial optimization (MCO) of IMRT fluence profiles and beam angles (FMO + BAO).18 Erasmus‐iCycle automatically generates a single Pareto‐optimal radiotherapy treatment plan. As it only optimizes pencil‐beam intensities, the system was originally integrated with the commercial Monaco TPS (Elekta AB, Stockholm, Sweden) to convert generated plans into clinically deliverable plans for C‐arm linacs.19, 20
In this paper, we propose and validate a novel, fully automated treatment planning solution for a CyberKnife® equipped with the InCiseTM 2 MLC (Accuray Inc., Sunnyvale, USA). Plan generation is performed fully outside the clinical treatment planning system, using Erasmus‐iCycle for pencil‐beam based FMO and BAO,18, 19, 20 followed by MLC segment generation aimed at close reproduction of the pencil‐beam optimized 3D dose distributions. The applied segmentation algorithm is fully compatible with all characteristics of the InCiseTM 2 MLC.21, 22 All pencil‐beam, MLC segment, and final dose distributions were calculated with a stand‐alone version of the commercial dose calculation engine. As automated plan generation includes BAO, the plans are denoted “AUTO BAO” in the remainder of the paper. Generated BAO plans could in principle be delivered on a CyberKnife®, as the commercial dose calculation is used and the InCiseTM 2 MLC is modeled accordingly. However, the applied software has no FDA clearance, and also according to the recently issued MDR (Medical Device Regulations), the system can currently not be applied for clinical treatment.
The main aim of this paper was to develop a new automated treatment planning pipeline, independent of the CyberKnife® supplier, for generation of deliverable plans and to evaluate whether it could in principle replace the current manual planning, with the well‐known plan quality issues of the latter and involved workload. Apart from dosimetric plan quality also delivery efficiency [Monitor Units (MU), number of beams, number of segments] and delivery times were evaluated.
For validation, the novel autoplanning workflow was first configured for prostate SBRT. For a group of 33 prostate SBRT patients, their AUTO BAO plan was compared to a reference plan (“REF”) that was manually generated with the commercial TPS. Each REF plan was also compared to a corresponding plan with automated FMO for the (fixed, patient‐specific) beam angles in the REF plan (“AUTO RB”: AUTO Reference Beams).
2. MATERIALS AND METHODS
2.1. Patient data and planning protocol
In this study, contoured planning CT‐scans of 33 patients treated with robotic radiotherapy for low‐ to intermediate‐stage prostate cancer were used. Patients were scanned in head‐first, supine position, with an average slice thickness of 1.55 mm [min: 1.50 mm, max: 3.00 mm], and an average pixel spacing of 0.97 mm [min: 0.80 mm, max: 0.98 mm] in X and Y directions. The patients were irradiated with a hypofractionated SBRT protocol, delivering 38 Gy in 4 fractions, and featuring highly heterogeneous PTV dose distributions (mimicking HDR brachytherapy).23, 24 For the PTV, a uniform volume expansion of 3 mm of the CTV was used. The average PTV volume was 70.2 cm3 (41.7–128.5 cm3). The PTV coverage objective was defined as 95% of the PTV volume should receive the prescribed dose. The clinical dose–volume constraints for this protocol are listed in Table I. The intention for rectum and bladder was to keep the near‐maximum doses () below 32.3 and 38 Gy respectively. However, when considered infeasible, the 1 cc constraint could be relaxed to 1.2 and 1.5 cc for rectum and bladder respectively.
Table I.
Clinical dose–volume constraints.
| Structure | Constraint | |||
|---|---|---|---|---|
| PTV |
|
|
||
| Urethra |
|
|
||
|
|
|
|||
|
|
|
|||
| Rectum |
|
|
||
|
|
|
|||
| Rectum mucosa |
|
|
||
| Bladder |
|
|
||
|
|
|
|||
| Femoral heads |
|
|
2.2. Reference plans (REF)
Attention was paid to using high‐quality, manually generated REF plans for validation of the new autoplanning pipeline. All REF plans were generated by a single experienced medical physicist, using manual iterative trial‐and‐error planning with the VOLOTM optimizer as introduced in Precision v. 2.0.0.0. (Accuray Inc., Sunnyvale, USA), in the absence of time pressure. Prior to generation of final plans, the physicist spent ample time (in the order of weeks) to develop a strategy for efficient generation of acceptable, high‐quality REF plans. The option to preselect a randomized and spatially distributed subset of nodes prior to optimization was not used for the generation of the REF plans. Instead, optimization started with all possible nodes available in the prostate robot motion path to use all available degrees of freedom for obtaining highest quality REF plans.
The REF plans were originally generated for planning study to validate the new VOLOTM optimizer by comparisons with clinical plans generated with the sequential optimization approach, both implemented in the commercial TPS. This study, by Giżyńska et al.,25 showed that the REF plans were highly superior to the clinical plans, both in terms of dosimetric plan quality as in plan deliverability. Also, several other studies reported enhanced plan quality when using the VOLOTM optimizer instead of the sequential optimization approach.26, 27, 28
2.3. AUTO plans
In contrast to manual Pareto navigation based MCO, Erasmus‐iCycle based MCO entails automated generation of a single Pareto‐optimal plan for each patient. Plan generation is based on a planning protocol specific “wish‐list” that is used for all patients treated according to the protocol. The wish‐list contains hard planning constraints and prioritized planning objectives.18 For this study, a dedicated wish‐list was constructed for the clinical prostate SBRT planning protocol, considering the planning constraints in Table I. The wish‐list can be found in Appendix A. Erasmus‐iCycle can handle DVH constraints directly. Originally the DVH constraints could only be used by approximation,29 but more recently also with high accuracy.30 Due to the induced complexity of using DVH criteria, only hard clinical DVH constraints are included in the FMO wish‐list. For the wish‐list configuration, five patients (of the 33 included patients) were used for training, and five extra patients for testing (fine‐tuning of the wish‐list). In this study, BAO meant generation of patient‐specific 25‐beam configurations.
Both FMO and segmentation were performed using a pencil‐beam resolution of 3 mm in the direction of the leaves and 3.85 mm perpendicular to the leaves at 800 mm SAD (leaf width of the InCiseTM 2 MLC). Pencil‐beam and segment dose depositions were calculated with a stand‐alone version of CyberKnife’s dose engine, provided by Accuray Inc. For the segmentation phase of this study, a MU penalty weight equal to 3 [Eq. (2) in Schipaanboord et al.21] was used. A degradation tolerance of 0.25% per objective (Table I) was used for the segment reduction method, which iteratively removes low contribution segments after the segmentation as long as the objective degradation is within the specified tolerance. The segmentation algorithm does not necessarily utilize all candidate beams provided for FMO, therefore the final deliverable AUTO RB plans may have fewer beams than the provided candidate beamset of the corresponding REF plan.
2.4. Plan evaluation and comparison
A PTV coverage of 95% was aimed for, however, for some patients this was not feasible due to limiting OAR constraints (Table I). Prior to comparing treatment planning strategies (REF vs AUTO BAO and AUTO RB), all three plans of a patient were normalized to exactly the same PTV coverage to minimize bias in dose delivery comparisons for healthy tissues, generally 95%. If 95% coverage was not feasible due to limiting OAR constraints for one or more treatment plans of a patient, all plans for that patient were normalized to the plan with the lowest PTV coverage to avoid inducing OAR constraint violations by normalizing to a higher PTV coverage.
AUTO plans were compared with REF plans using dosimetric plan parameters applied in clinical practice, Dose Volume Histograms (DVHs), visual inspection of the dose distributions, Conformation Number,31 numbers of beam directions, numbers of MLC segments, numbers of MU per fraction, and estimated treatment delivery times, calculated with a stand‐alone treatment time estimator provided by Accuray Inc. The estimated treatment time (ETT) includes beam‐on time, robot movements, changing of apertures, and imaging, while excluding patient setup time.
Wilcoxon signed‐rank tests for paired data were performed to assess statistical significance (P < 0.05) of differences between AUTO plans and manually generated REF plans.
2.5. Computation times
Computation times for AUTO plans were measured for 10 patients on an Intel Xeon Gold 6248 @ 2.5 GHz, containing 40 cores and with 386 GB of memory.
3. RESULTS
3.1. Plan comparisons
The population averaged DVHs in Fig. 1 show high similarity for the three planning approaches, with small advantages for autoplanning compared to REF for the higher urethra doses and in the intermediate dose range for rectum and bladder (especially for AUTO RB). An example dose distribution for REF and AUTO BAO is shown in Fig. 2.
Fig. 1.
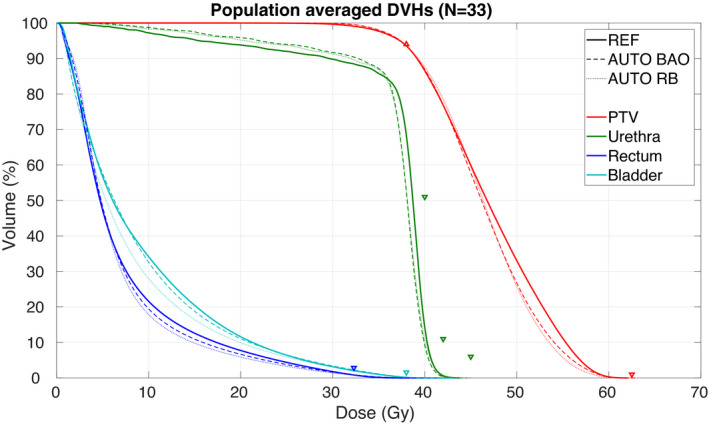
Population averaged PTV, rectum, bladder, and urethra Dose Volume Histograms (DVHs) for the reference plans (REF), the AUTO plans with beam angle optimization (AUTO BAO), and the AUTO plans with the reference beam geometry (AUTO RB). Clinical constraints are denoted with triangles, see Table I.
Fig. 2.
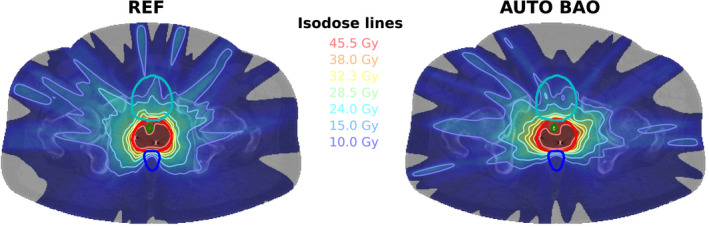
An example dose distribution for REF (left) and AUTO BAO (right). Depicted structures: PTV (red), Urethra (green), Rectum (dark blue), and Bladder (light blue).
The dosimetric plan parameter comparisons presented in Fig. 3 confirm the overall similarity between AUTO plans and REF plans. Depending on the parameter, small overall advantages for AUTO plans or REF plans were seen. Some patients demonstrate differences that could possibly be clinically relevant, sometimes in favor of AUTO, sometimes in favor of REF. The upper parts of Tables II and III present overviews of differences between dosimetric plan parameters in REF plans and AUTO BAO plans (Table II) or AUTO RB plans (Table III). Although many of the differences in dosimetric plan parameters are statistically significant, they are small from the clinical point of view, sometimes in favor of AUTO and for other parameters in favor of REF.
Fig. 3.
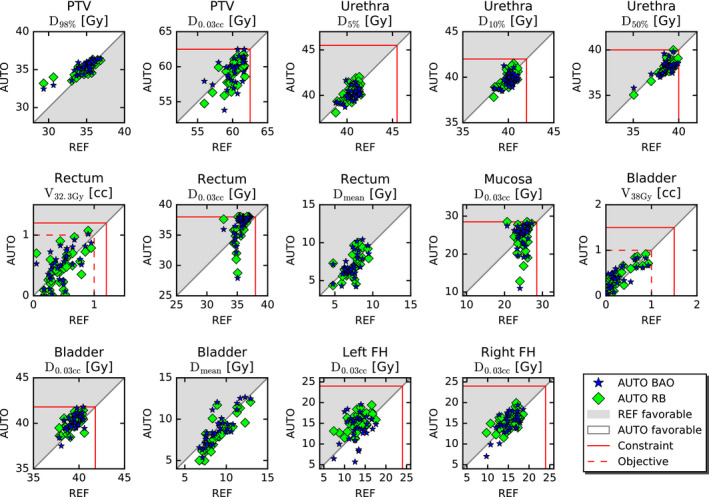
Comparisons of REF, AUTO BAO, and AUTO RB regarding dosimetric plan parameters. Every marker represents a plan parameter comparison for one of the 33 study patients. Red lines show treatment planning aims. FH: Femoral head. See Section 2.A for differences between constraint and objective levels.
Table II.
Plan parameter comparisons for AUTO BAO and REF plans.
| REF | AUTO BAO | AUTO BAO ‐ REF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (min, max) | Mean | (min, max) |
|
(min, max) | P | |||
| PTV |
|
34.7 | (29.3, 36.8) | 35.3 | (32.5, 36.5) | 0.6 | (−1.1, 3.1) | <0.001 | |
|
|
60.2 | (56.0, 61.8) | 59.5 | (53.8, 62.5) | −0.7 | (−5.0, 2.6) | 0.03 | ||
| Urethra |
|
40.5 | (38.8, 41.5) | 40.1 | (38.7, 41.7) | −0.4 | (−1.5, 1.8) | 0.005 | |
|
|
40.0 | (38.4, 41.1) | 39.7 | (38.5, 41.3) | −0.4 | (−1.5, 1.7) | 0.005 | ||
|
|
38.6 | (35.1, 39.9) | 38.1 | (35.8, 39.8) | −0.6 | (−1.8, 1.2) | <0.001 | ||
| Rectum |
|
0.5 | (0.0, 1.0) | 0.5 | (0.0, 1.0) | 0.0 | (−0.5, 0.5) | 0.5 | |
|
|
35.5 | (32.8, 37.0) | 36.4 | (27.9, 38.0) | 0.8 | (−7.2, 3.2) | <0.001 | ||
|
|
7.2 | (4.7, 9.5) | 7.1 | (4.2, 10.4) | −0.2 | (−3.6, 2.4) | 0.4 | ||
| Mucosa |
|
24.8 | (20.7, 27.2) | 24.7 | (11.0, 28.1) | −0.0 | (−13.1, 6.8) | 0.3 | |
| Bladder |
|
0.3 | (0.0, 0.9) | 0.4 | (0.0, 0.8) | 0.1 | (−0.3, 0.4) | 0.001 | |
|
|
39.4 | (37.8, 40.6) | 40.0 | (37.5, 41.8) | 0.6 | (−1.1, 2.0) | <0.001 | ||
|
|
9.1 | (6.8, 12.9) | 8.9 | (5.4, 12.6) | −0.2 | (−2.1, 3.1) | 0.1 | ||
| Entrance |
|
17.3 | (14.2, 19.9) | 18.0 | (15.3, 20.1) | 0.7 | (−2.8, 4.2) | 0.02 | |
| Left FH |
|
13.2 | (7.4, 17.6) | 13.9 | (5.7, 19.6) | 0.7 | (−6.9, 7.8) | 0.2 | |
| Right FH |
|
15.1 | (9.7, 18.6) | 15.0 | (7.0, 19.4) | −0.1 | (−3.3, 4.3) | 0.8 | |
| Conformality | 0.82 | (0.75, 0.88) | 0.79 | (0.75, 0.87) | −0.03 | (−0.07, 0.02) | <0.001 | ||
| Beams | 48.3 | (37.0, 59.0) | 24.7 | (22.0, 25.0) | −23.6 | (−34.0, −12.0) | <0.001 | ||
| Segments | 98.9 | (54.0, 167.0) | 59.5 | (40.0, 95.0) | −39.4 | (−115.0, 22.0) | <0.001 | ||
| MU/fx [1000] | 6.1 | (4.9, 8.9) | 6.2 | (4.9, 8.3) | 0.1 | (−2.7, 3.2) | 0.5 | ||
| ETT [min] | 22.3 | (17.0, 32.0) | 17.1 | (14.1, 22.0) | −5.3 | (−15.6, 1.0) | <0.001 | ||
Doses (Dxx) are presented in Gy and volume (Vxx) in cc. Entrance dose was evaluated using of a ring structure of 3 cm thickness inside the patient's external contour. FH: Femoral head, MU/fx: Monitor Units per fraction, ETT: Estimated treatment time.
Bold indicates statistically significant P‐value
Table III.
Plan parameter comparisons for AUTO RB and REF plans.
| REF | AUTO RB | AUTO RB ‐ REF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (min, max) | Mean | (min, max) |
|
(min, max) | P | |||
| PTV |
|
34.7 | (29.3, 36.8) | 35.1 | (33.2, 36.3) | 0.4 | (−1.1, 3.8) | 0.02 | |
|
|
60.2 | (56.0, 61.8) | 59.1 | (54.7, 61.8) | −1.1 | (−3.4, 1.9) | <0.001 | ||
| Urethra |
|
40.5 | (38.8, 41.5) | 40.3 | (38.1, 42.0) | −0.2 | (−1.5, 1.3) | 0.1 | |
|
|
40.0 | (38.4, 41.1) | 39.8 | (37.8, 41.5) | −0.2 | (−1.2, 1.0) | 0.03 | ||
|
|
38.6 | (35.1, 39.9) | 38.1 | (35.1, 40.0) | −0.6 | (−1.4, 0.6) | <0.001 | ||
| Rectum |
|
0.5 | (0.0, 1.0) | 0.4 | (0.0, 1.1) | −0.0 | (−0.5, 0.7) | 0.3 | |
|
|
35.5 | (32.8, 37.0) | 36.4 | (28.8, 38.0) | 0.8 | (−6.3, 4.9) | 0.004 | ||
|
|
7.2 | (4.7, 9.5) | 6.8 | (4.4, 10.0) | −0.4 | (−3.3, 2.5) | 0.03 | ||
| Mucosa |
|
24.8 | (20.7, 27.2) | 24.0 | (12.8, 28.5) | −0.8 | (−11.2, 7.8) | 0.3 | |
| Bladder |
|
0.3 | (0.0, 0.9) | 0.4 | (0.1, 0.9) | 0.1 | (−0.2, 0.3) | <0.001 | |
|
|
39.4 | (37.8, 40.6) | 40.1 | (38.5, 41.4) | 0.7 | (−1.6, 2.2) | <0.001 | ||
|
|
9.1 | (6.8, 12.9) | 8.2 | (5.0, 12.1) | −0.9 | (−2.7, 2.8) | <0.001 | ||
| Entrance |
|
17.3 | (14.2, 19.9) | 17.2 | (12.9, 21.4) | −0.0 | (−4.4, 3.4) | 0.9 | |
| Left FH |
|
13.2 | (7.4, 17.6) | 15.4 | (11.3, 19.4) | 2.2 | (−1.7, 5.7) | <0.001 | |
| Right FH |
|
15.1 | (9.7, 18.6) | 15.6 | (11.5, 19.9) | 0.5 | (−3.3, 4.2) | 0.2 | |
| Conformality | 0.82 | (0.75, 0.88) | 0.78 | (0.72, 0.84) | −0.04 | (−0.07, −0.01) | <0.001 | ||
| Beams | 48.3 | (37.0, 59.0) | 38.0 | (29.0, 49.0) | −10.4 | (−23.0, −2.0) | <0.001 | ||
| Segments | 98.9 | (54.0, 167.0) | 61.5 | (40.0, 105.0) | −37.5 | (−118.0, 37.0) | <0.001 | ||
| MU/fx [×1000] | 6.1 | (4.9, 8.9) | 6.2 | (4.7, 7.4) | 0.1 | (−2.5, 1.8) | 0.2 | ||
| ETT [min] | 22.3 | (17.0, 32.0) | 18.6 | (15.0, 24.3) | −3.7 | (−11.7, 4.0) | <0.001 | ||
Doses (Dxx) presented in Gy and volumes (Vxx) in cc. Entrance dose was evaluated using of a ring structure of 3 cm thickness inside the patient's external contour. FH: Femoral head, MU/fx: Monitor Units per fraction, ETT: Estimated treatment time.
Bold indicates statistically significant P‐value
Results for nondosimetric parameters are presented in the bottom sections of Tables II and III in Fig. 4. A clinically relevant reduction in estimated treatment time was observed for the AUTO plans compared to the REF (AUTO BAO: −5.3 min. [−15.6, 1.0], AUTO RB: −3.7 min. [−11.7, 4.0]). This was related to reductions in the numbers of beams (AUTO BAO: −23.6 [−34, −12], AUTO RB: −10.4 [−23, −2]) and the numbers of segments (AUTO BAO: −39.4 [−115, −12], AUTO RB: −37.5 [−118, −37]), whereas no significant differences for the number of MU per fraction were observed.
Fig. 4.
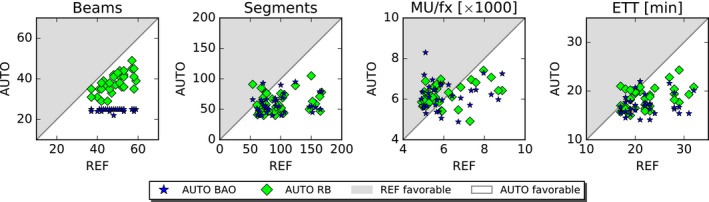
Nondosimetric plan parameters of the AUTO plans compared to corresponding REF plan parameters. Every marker represents a plan parameter comparison for one of the 33 study patients. MU/fx: Monitor Units per fraction, ETT: Estimated treatment time.
3.2. Computation times
Table IV shows autoplanning mean computations times with ranges, divided in (a) generation of the pencil‐beam matrices together with the optimization of the FMO dose distributions (PB + FMO) and, (b) the subsequent segmentation of the FMO dose distributions (Segmentation).
Table IV.
Planning and computation times in minutes calculated over the first 10 patients.
| Approach | PB + FMO | Segmentation | Total | |||
|---|---|---|---|---|---|---|
| Mean | (min, max) | Mean | (min, max) | Mean | (min, max) | |
| REF (manual) | 50 | (10, 170) | ||||
| AUTO BAO | 621 | (529, 742) | 16 | (13, 19) | 637 | (542, 761) |
| AUTO RB | 80 | (53, 169) | 18 | (14, 24) | 98 | (67, 193) |
The reported REF planning time is manual hands‐on time, while the reported AUTO times are fully automated calculation times without manual interaction. PB + FMO: Calculation of pencil‐beams + Fluence Map Optimization.
4. DISCUSSION
In this study, we have proposed a fully automated treatment planning workflow for a robotic CyberKnife® unit equipped with the InCiseTM 2 MLC, and validated it for prostate SBRT by comparison of generated AUTO BAO plans with high‐quality, manually generated reference plans (REF). The AUTO BAO plans are optimized fully independently of the commercial TPS, using in‐house developed applications for integrated multicriterial pencil‐beam‐based beam angle and fluence map optimization,18, 20 and subsequent generation of MLC segments.21, 22 Although practically not yet possible because of plan import restrictions, the plans are in principle deliverable at clinical CyberKnife® units. The AUTO BAO prostate SBRT plans were clinically acceptable with a quality equivalent to the REF plans. However, as no manual fine‐tuning of AUTO plans was needed, the quality of these plans was independent of manual planners and the workload was virtually zero. AUTO BAO also had shorter delivery times (23% reduction, 5.3 min.) and vastly reduced numbers of beams (24.7 vs 48.3) and segments (59.5 vs 98.9).
To the best of our knowledge, this is the first fully independent system for automated generation of deliverable plans that combines automated noncoplanar BAO, FMO, and segmentation all together. Automated generation of clinically deliverable plans was developed for other systems, but final plan generation was always performed using the commercial TPS. Deliverable plans were created but the planning workflow required manual tweaking during plan optimization or did not include noncoplanar BAO. For example, Erasmus‐iCycle was integrated with Monaco (Elekta AB, Stockholm, Sweden)19, 20 for treatment with C‐arm linacs, and to MultiPlan (Accuray Inc., Sunnyvale, USA)32 for treatment with a CyberKnife® in combination with the IRISTM collimator. The 4π planning approach relies on importing optimized beam angles into Eclipse (Varian Medical System, Palo Alto, USA), followed by conventional treatment planning.33, 34, 35, 36 The Expedited Constrained Hierarchical Optimization (ECHO) system also uses Eclipse to generate a final plan, the optimized fluence maps are imported and then leaf sequencing is performed within Eclipse.15 The ASEQ method used for online replanning creates deliverable plans with the use of the Monte Carlo dose engine (GPUMCD) from Elekta AB, but requires manual tweaking during plan optimization and uses predefined clinical beam configurations.37, 38, 39, 40
The use of column‐generation in radiotherapy treatment planning was first proposed by Romeijn et al.41 to solve the Direct Aperture Optimization (DAO) problem. The 4π planning approach42, 43 also uses a column‐generation approach, based on the formulation proposed by Romeijn et al., but uses column‐generation to solve the BAO/FMO problem. In the proposed autoplanning workflow for CyberKnife® with MLC, the BAO/FMO problem is solved using a multicriterial optimization as implemented in Erasmus‐iCycle.18 Then, a column‐generation method, inspired by the approach by Romeijn et al., was used to solve the segmentation problem by mimicking the 3D FMO dose distribution.21, 22 This two‐step approach of BAO/FMO followed by segmentation turns out to be more flexible for the multicriteria optimization.
Computation times for AUTO BAO plans were on average 637 min. While this may seem long, important to realize is that no manual hands‐on planning or manual corrections of the computed plans were applied. In a clinical context, this would mean that a deliverable plan is ready within a day of contour approval by the treating physician. In our clinical practice, this has always been a requirement for application of Erasmus‐iCycle based automated plan generation for C‐arm linacs (although current plan generation is much faster). Contributing most to the computation time was the applied integrated, iterative BAO which is computationally expensive for large numbers of beams. This BAO approach was chosen because it has been shown to provide good quality treatment plans in previous studies.18, 32, 44, 45, 46 Alternatively, the proposed workflow could also be combined with a BAO approach that selects beam angles prior to FMO optimization. This would avoid the need for multiple FMO iterations, which would reduce the computation time. Instead of the patient‐specific beam angle optimization, a predefined set of beam angles (class‐solution) could be used for all patients, for example, the noncoplanar beam angle class‐solution proposed by Rossi et al.47 Furthermore, our current research implementation utilizes serial calculation of the pencil‐beams and the final dose calculations (included in both FMO and segmentation). Parallelization of these calculation steps could reduce the computation time substantially.
The presented comparison of AUTO RB plans with REF plans allowed us to compare autoplanning with manual planning without a bias of different beam angles; the AUTO RB plans indicate what performance could be achieved with the CyberKnife® system for each patient when using a different optimization approach for the same input beam angles (as used in the REF plan). As shown in Table III, not all input angles were always used in the final AUTO RB plans (on average 38.0 of the 48.3 beams in REF). Nevertheless, quality of the AUTO RB plans was similar to the REF plan quality. Apart from the reduced number of beams, this was obtained with also a substantially lower number of segments and a significantly reduced delivery time (Table III). In the intermediate dose range, AUTO RB plans are slightly favorable over AUTO BAO plans (Fig. 1). This is attributed to the lower numbers of beams used in AUTO BAO (24.7 vs 38.0). For AUTO BAO our aim was to obtain a quality that was comparable to REF. For example, 38 beams in AUTO BAO would have further enhanced the quality of the AUTO BAO plans, but at the cost of large increases in calculation time.
In this study, we have introduced a novel automated treatment planning pipeline for CyberKnife® SBRT and we validated it for prostate cancer. In an ongoing study, the new workflow is being investigated for lung SBRT, another type of treatment that is frequently performed with the CyberKnife®.
Recently, the RATING framework with guidelines for performing high‐quality treatment planning studies has been published.48 There is also a score sheet attached to the framework to get a quantitative impression on the quality of treatment planning papers. According this sheet, our study scored 92/100%. The filled‐out sheet is provided in Appendix B.
5. CONCLUSIONS
A new vendor‐independent workflow for fully automated generation of deliverable, high‐quality CyberKnife plans was proposed, including patient‐specific beam angle optimization (BAO). Compared to manual planning with the commercial TPS in absence of time pressure, dosimetric plan quality for prostate SBRT was similar, while fraction delivery times reduced by 5.3 min (from 22.3 to 17.1 min) due to large reductions in beam and segment numbers.
CONFLICT OF INTEREST
The authors have no relevant conflicts of interest to disclose.
Supporting information
Data S1. Filled‐out RATING score sheet
ACKNOWLEDGMENTS
This work was in part funded by a research grant from Accuray Inc., Sunnyvale, USA. Erasmus MC Cancer Institute also has a collaboration agreement with Elekta AB, Stockholm, Sweden.
APPENDIX A.
Table A1.
The wish‐list used to automatically generate the FMO plans using Erasmus‐iCycle, containing a list of constraints and a list of prioritized planning objectives.
| Constraints | |||
|---|---|---|---|
| Priority | Volume | Dose metric | Limit (Gy) |
| PTV | Dmax | 61.5 | |
| UrethraPlan | D5% | 45 | |
| D10% | 42 | ||
| Dmean | 40 | ||
| Rectum | Dmax | 38 | |
| D1cc | 32.3 | ||
| Rectum mucosa | Dmax | 27 | |
| Bladder | Dmax | 41.8 | |
| D1cc | 38 | ||
| Penile bulb | Dmax | 1.5 | |
| Ring PTV 2–3cm | Dmax | 25 | |
| Skin dose | Dmax | 20 | |
| Shell 3 mm | Dmax | 38 | |
| Shell 3 cm | Dmax | 20 | |
| Shell 5 cm | Dmax | 20 | |
| Objectives | |||||
|---|---|---|---|---|---|
| Priority | Volume | Dose metric | Goal | Sufficient | Parameters |
| 1 | PTV ‐ OARs | LTCP | 0.01 | 0.01 | = 37 Gy, = 0.90 |
| 2 | PTV | LTCP | 0.25 | 0.25 | = 37 Gy, = 0.60 |
| 3 | UrethraPlan | Dmean | 39 Gy | 38 Gy | |
| 4 | LTCP | 0.50 | = 40 Gy, = −0.50 | ||
| 5 | Rectum | LTCP | 0.00 | = 27 Gy, = −0.20 | |
| 6 | Bladder | LTCP | 0.00 | = 31 Gy, = −0.20 | |
| 7 | Dose bath | Dmax | 15 Gy | ||
| 8 | Femoral heads | Dmax | 24 Gy | ||
The optimization starts with optimizing on the PTV to attain sufficient PTV coverage, while respecting all constraints from the constraints list. Once an objective is optimized, it is added as an additional constraint to the constraint list and then the next objective will be optimized. The relative ranking of the objectives is based on clinical importance of the structures. A detailed description of the Erasmus‐iCycle workflow is provided in Breedveld et al., 2012.18 UrethraPlan is defined as the volume of the urethra within the PTV.
APPENDIX B.
Filled‐out RATING score sheet attached.
REFERENCES
- 1.Nelms BE, Robinson G, Markham J, et al. Variation in external beam treatment plan quality: an inter‐ institutional study of planners and planning systems. Pract Radiat Oncol. 2012;2:296–305. [DOI] [PubMed] [Google Scholar]
- 2.Marino C, Villaggi E, Maggi G, et al. A feasibility dosimetric study on prostate cancer. Strahlentherapie und Onkologie. 2015;191:573–581. [DOI] [PubMed] [Google Scholar]
- 3.Giglioli FR, Strigari L, Ragona R, et al. Lung stereotactic ablative body radiotherapy: a large scale multi‐institutional planning comparison for interpreting results of multi‐institutional studies. Phys Med. 2016;32:600–606. [DOI] [PubMed] [Google Scholar]
- 4.Berry SL, Boczkowski A, Ma R, Mechalakos J, Hunt M. Interobserver variability in radiation therapy plan output: results of a single‐institution study. Pract Radiat Oncol. 2016;6:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breedveld S, Craft D, van Haveren R , Heijmen B. Multi‐criteria optimization and decision‐making in radiotherapy. Eur J Oper Res. 2019;277:1–19. [Google Scholar]
- 6.Heijmen B, Voet P, Fransen D, et al. Fully automated, multi‐criterial planning for Volumetric Modulated Arc Therapy ‐ An international multi‐center validation for prostate cancer. Radiother Oncol. 2018;128:343–348. [DOI] [PubMed] [Google Scholar]
- 7.Purdie TG, Dinniwell RE, Fyles A, Sharpe MB. Automation and intensity modulated radiation therapy for individualized high-quality tangent breast treatment plans. Int J Radiat Oncol Biol Phys. 2014;90:688–695. 10.1016/j.ijrobp.2014.06.056 [DOI] [PubMed] [Google Scholar]
- 8.Fogliata A, Belosi F, Clivio A, et al. On the pre‐clinical validation of a commercial model‐based optimisation engine: application to volumetric modulated arc therapy for patients with lung or prostate cancer. Radiother Oncol. 2014;113:385–391. [DOI] [PubMed] [Google Scholar]
- 9.Tol JP, Delaney AR, Dahele M, Slotman BJ, Verbakel WF. Evaluation of a knowledge‐based planning solution for head and neck cancer. Int J Radiat Oncol Biol Phys. 2015;91:612–620. [DOI] [PubMed] [Google Scholar]
- 10.Hansen CR, Bertelsen A, Hazell I, et al. Automatic treatment planning improves the clinical quality of head and neck cancer treatment plans. Clin Transl Radiat Oncol. 2016;1:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen CR, Nielsen M, Bertelsen AS, et al. Automatic treatment planning facilitates fast generation of high‐quality treatment plans for esophageal cancer. Acta Oncol. 2017;56:1495–1500. 10.1080/0284186x.2017.1349928 [DOI] [PubMed] [Google Scholar]
- 12.Hussein M, South CP, Barry MA, et al. Clinical validation and benchmarking of knowledge‐based IMRT and VMAT treatment planning in pelvic anatomy. Radiother Oncol. 2016;120:473–479. [DOI] [PubMed] [Google Scholar]
- 13.Hussein M, Heijmen BJM, Verellen D, Nisbet A. Automation in intensity modulated radiotherapy treatment planning ‐ a review of recent innovations. Br J Radiol. 2018;91:20180270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrazzo L, Meattini I, Arilli C, et al. Auto‐planning for VMAT accelerated partial breast irradiation. Radiother Oncol. 2019;132:85–92. [DOI] [PubMed] [Google Scholar]
- 15.Zarepisheh M, Hong L, Zhou Y, et al. Automated intensity modulated treatment planning: the expedited constrained hierarchical optimization (ECHO) system. Med Phys. 2019;46:2944–2954. 10.1002/mp.13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giglioli FR, Garibaldi C, Blanck O, et al. Dosimetric multicenter planning comparison studies for stereotactic body radiation therapy: methodology and future perspectives. Int J Radiat Oncol Biol Phys. 2020;106:403–412. [DOI] [PubMed] [Google Scholar]
- 17.Oud M, Kolkman‐Deurloo IK, Mens JW, et al. Fast and fully‐automated multi‐criterial treatment planning for adaptive HDR brachytherapy for locally advanced cervical cancer. Radiother Oncol. 2020;148:143–150. [DOI] [PubMed] [Google Scholar]
- 18.Breedveld S, Storchi P, Voet P, Heijmen B. iCycle: Integrated, multicriterial beam angle, and profile optimization for generation of coplanar and noncoplanar IMRT plans. Med Phys. 2012;39:951–963. [DOI] [PubMed] [Google Scholar]
- 19.Voet P, Dirkx M, Breedveld S, Fransen D, Levendag P, Heijmen B. Towards fully automated multi‐criterial plan generation: a prospective clinical study. Int J Radiat Oncol Biol Phys. 2013;85:866–872. [DOI] [PubMed] [Google Scholar]
- 20.Voet PWJ, Dirkx MLP, Breedveld S, et al. Fully automated volumetric modulated arc therapy plan generation for prostate cancer patients. Int J Radiat Oncol Biol Phys. 2014;88:1175–1179. 10.1016/j.ijrobp.2013.12.046 [DOI] [PubMed] [Google Scholar]
- 21.Schipaanboord BWK, Breedveld S, Rossi L, Keijzer M, Heijmen B. Automated prioritised 3D dose‐based MLC segment generation for step‐and‐shoot IMRT. Phys Med Biol. 2019;64:165013. 10.1088/1361-6560/ab1df9 [DOI] [PubMed] [Google Scholar]
- 22.Schipaanboord BWK, Heijmen B, Breedveld S. Accurate 3D‐dose‐based generation of MLC segments for robotic radiotherapy. Phys Med Biol. 2020;65:175011. [DOI] [PubMed] [Google Scholar]
- 23.Aluwini S, van Rooij P , Hoogeman M, et al. CyberKnife stereotactic radiotherapy as monotherapy for low‐to intermediate‐stage prostate cancer: early experience, feasibility and tolerance. J Endourol. 2010;24:865–869. [DOI] [PubMed] [Google Scholar]
- 24.Aluwini S, van Rooij P , Hoogeman M, Kirkels W, Kolkman‐Deurloo IK, Bangma C. Stereotactic body radiotherapy with a focal boost to the MRI‐visible tumor as monotherapy for low‐ and intermediate‐risk prostate cancer: early results. Radiat Oncol. 2013;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giżyńska MK, Rossi L, Toom W, et al. Largely reduced OAR doses, and planning and delivery times for challenging robotic SBRT cases, obtained with a novel optimizer. J Appl Clin Med Phys. 2021;22:35–47. 10.1002/acm2.13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeverino M, Marguet M, Zulliger C, et al. Novel inverse planning optimization algorithm for robotic radiosurgery: first clinical implementation and dosimetric evaluation. Phys Med. 2019;64:230–237. [DOI] [PubMed] [Google Scholar]
- 27.Schüler E, Lo A, Chuang CF, Soltys SG, Pollom EL, Wang L. Clinical impact of the VOLO optimizer on treatment plan quality and clinical treatment efficiency for CyberKnife. J Appl Clin Med Phys. 2020;21:38–47. 10.1002/acm2.12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calusi S, Doro R, Di Cataldo V, Cipressi S, Francolini G, Bonucci I, Livi L, Masi L. Performance assessment of a new optimization system for robotic SBRT MLC‐based plans. Physica Medica. 2020;71:31–38. 10.1016/j.ejmp.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 29.Breedveld S, van den Berg B , Heijmen B. An interior‐point implementation developed and tuned for radiation therapy treatment planning. Comput Optim Appl. 2017;68:209–242. [Google Scholar]
- 30.Breedveld S, Bennan ABA, Aluwini S, Schaart DR, Kolkman‐Deurloo IK, Heijmen BJM. Fast automated multi‐criteria planning for HDR brachytherapy explored for prostate cancer. Phys Med Biol. 2019;64205002. [DOI] [PubMed] [Google Scholar]
- 31.van’t Riet A , Mak AC, Moerland MA, Elders LH, van der Zee W . A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys. 1997;37:731–736. [DOI] [PubMed] [Google Scholar]
- 32.Rossi L, Sharfo AW, Aluwini S, Dirkx M, Breedveld S, Heijmen B. First fully automated planning solution for robotic radiosurgery – comparison with automatically planned volumetric arc therapy for prostate cancer. Acta Oncol. 2018;57:1490–1498. 10.1080/0284186x.2018.1479068 [DOI] [PubMed] [Google Scholar]
- 33.Woods K, Nguyen D, Tran A, et al. Viability of non‐coplanar VMAT for liver SBRT as compared to coplanar VMAT and beam orientation optimized 4π IMRT. Adv Radiat Oncol. 2016;1:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran A, Zhang J, Woods K, et al. Treatment planning comparison of IMPT, VMAT and 4π radiotherapy for prostate cases. Radiat Oncol. 2017;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu VY, Landers A, Woods K, et al. A prospective 4π radiation therapy clinical study in recurrent high‐grade glioma patients. Int J Radiat Oncol Biol Phys. 2018;101:144–151. 10.1016/j.ijrobp.2018.01.048 [DOI] [PubMed] [Google Scholar]
- 36.Murzin VL, Woods K, Moiseenko V, et al. 4π plan optimization for cortical‐sparing brain radiotherapy. Radiother Oncol. 2018;127:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kontaxis C, Bol GH, Lagendijk JJ, Raaymakers BW. Towards adaptive IMRT sequencing for the MR‐linac. Phys Med Biol. 2015;60:2493–2509. [DOI] [PubMed] [Google Scholar]
- 38.Kontaxis C, Bol GH, Lagendijk JJ, Raaymakers BW. A new methodology for inter‐ and intrafraction plan adaptation for the MR‐linac. Phys Med Biol. 2015;60:7485–7497. [DOI] [PubMed] [Google Scholar]
- 39.Kontaxis C, Bol GH, Stemkens B, et al. Towards fast online intrafraction replanning for free‐breathing stereotactic body radiation therapy with the MR‐linac. Phys Med Biol. 2017;62:7233–7248. [DOI] [PubMed] [Google Scholar]
- 40.Kontaxis C, Bol GH, Kerkmeijer LGW, Lagendijk JJW, Raaymakers BW. Fast online replanning for interfraction rotation correction in prostate radiotherapy. Med Phys. 2017;44:5034–5042. [DOI] [PubMed] [Google Scholar]
- 41.Romeijn HE, Ahuja RK, Dempsey JF, Kumar A. A column generation approach to radiation therapy treatment planning using aperture modulation. SIAM J Optimiz. 2005;15:838–862. 10.1137/040606612 [DOI] [Google Scholar]
- 42.Dong P, Lee P, Ruan D, et al. 4π non‐coplanar liver SBRT: a novel delivery technique. Int J Radiat Oncol Biol Phys. 2013;85:1360–1366. 10.1016/j.ijrobp.2012.09.028 [DOI] [PubMed] [Google Scholar]
- 43.Dong P, Lee P, Ruan D, et al. 4π noncoplanar stereotactic body radiation therapy for centrally located or larger lung tumors. Int J Radiat Oncol Biol Phys. 2013;86:407–413. 10.1016/j.ijrobp.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 44.Voet P, Breedveld S, Dirkx M, Levendag P, Heijmen B. Integrated multi‐criterial optimization of beam angles and intensity pro_les for coplanar and non‐coplanar head and neck IMRT and implications for VMAT. Med Phys. 2012;39:4858–4865. [DOI] [PubMed] [Google Scholar]
- 45.Rossi L, Cambraia Lopes P, Marques Leitão J, et al. On the importance of individualized, non‐coplanar beam configurations in mediastinal lymphoma radiotherapy, optimized with automated planning. Front Oncol. 2021;11. 10.3389/fonc.2021.619929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharfo AWM, Rossi L, Dirkx MLP, Breedveld S, Aluwini S, Heijmen BJM. Complementing prostate SBRT VMAT with a two‐beam non‐coplanar IMRT class solution to enhance rectum and bladder sparing with minimum increase in treatment time. Front Oncol. 2021;11:620978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossi L, Breedveld S, Aluwini S, Heijmen B. Noncoplanar beam angle class solutions to replace time‐consuming patient‐specific beam angle optimization in robotic prostate stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:762–770. 10.1016/j.ijrobp.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 48.Hansen CR, Crijns W, Hussein M, et al. Radiotherapy Treatment plannINg study Guidelines (RATING): a framework for setting up and reporting on scientific treatment planning studies. Radiother Oncol. 2020;153:67–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Filled‐out RATING score sheet


