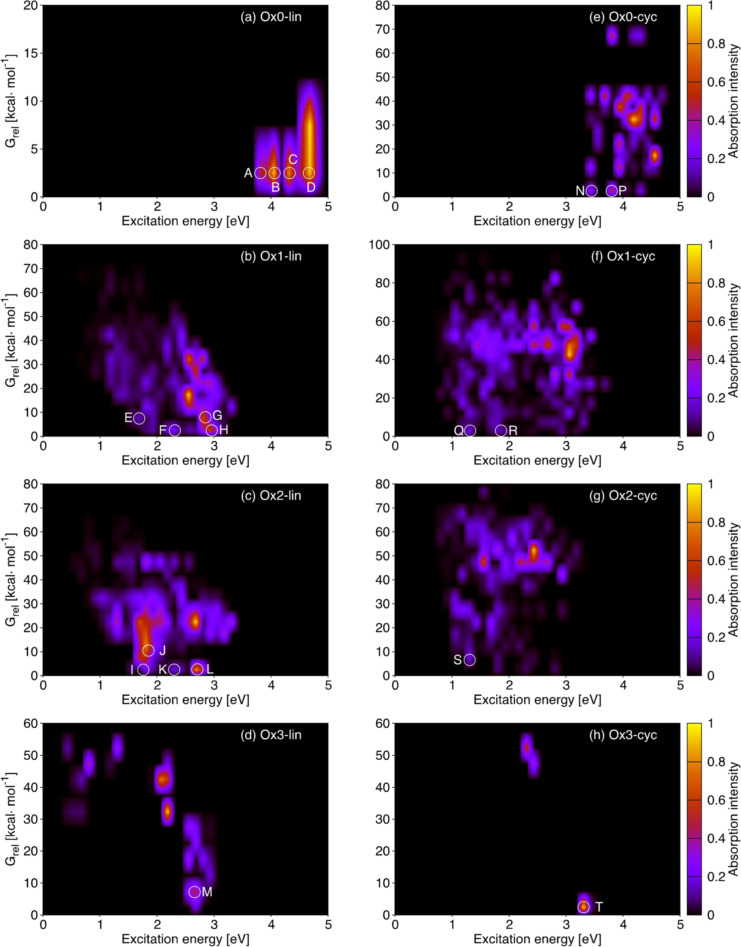
Figure 5.
S1‐S3 absorption properties (excitation energy, x axis, and oscillator strength, color bars) vs. stability (energy relative to the most stable compound of every group) of (a)–(d) linear and (e)–(h) cyclic library compounds grouped by oxidation state. The letters A‐T identify the most relevant features of stable compounds (low G rel).