Figure 1.
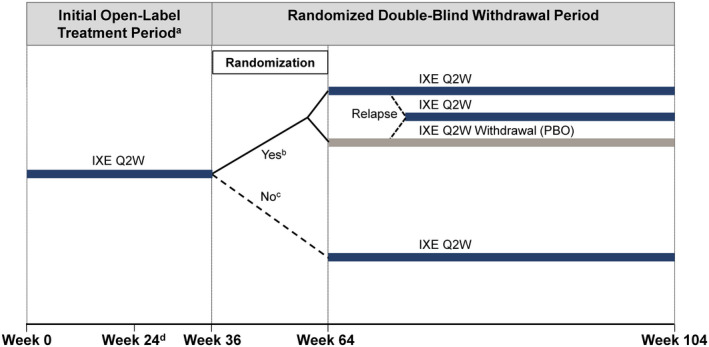
SPIRIT‐P3 study design. a Encompassed week 0 (study baseline) up to week 36. b Between weeks 36 and 64 (inclusive), patients treated with ixekizumab (IXE) every 2 weeks (Q2W) in whom minimal disease activity (MDA) was achieved for 4 consecutive visits for at least 36 weeks were eligible for randomization at the visit at which these criteria were met. Patients were randomized 1:1 to the ixekizumab every 2 weeks group or the ixekizumab withdrawal group. Patients remained in their treatment groups up to week 104 or until relapse (no longer met MDA), at which point they received ixekizumab every 2 weeks up to week 104. c Patients who did not meet the randomization eligibility criteria by week 64 continued to receive ixekizumab every 2 weeks uninterrupted up to week 104. d Patients in whom ≥20% improvement in tender joint count and swollen joint count at week 24 or at any subsequent visit through week 104, except from the point of randomization until the visit after relapse for patients in the randomized double‐blind withdrawal period, were discontinued from the study. PBO = placebo.
