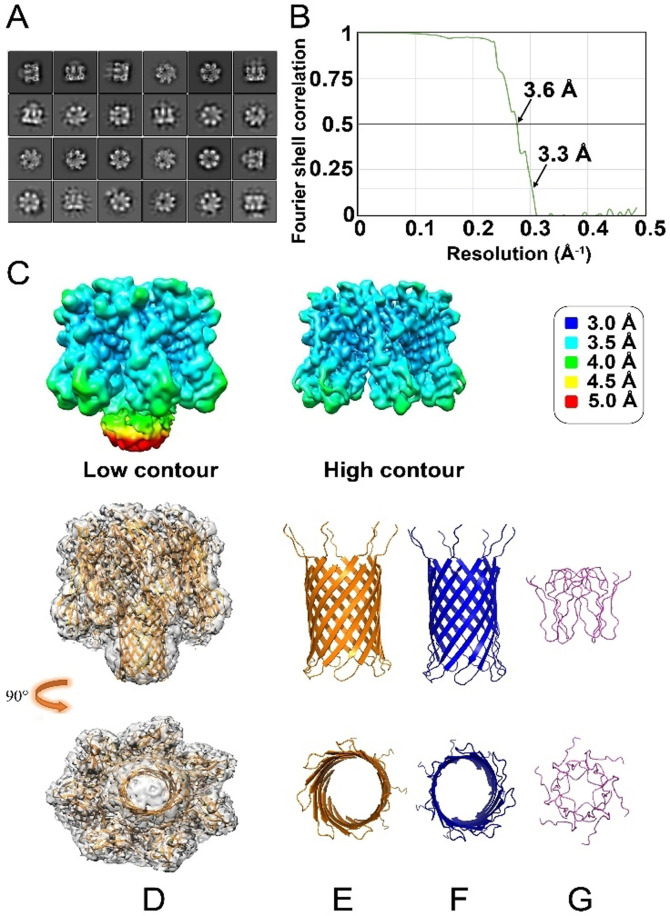
Figure 6.
Cryo‐EM data of Aβ42‐αHL. A) 2D classes show strong features for the αHL heptameric core, along with clear but more diffuse density for the structure formed by the Aβ42 sequences. B) The 3D average after the classification and refinement has an overall resolution of 3.3 Å at FSC=0.143 calculated by the gold‐standard Fourier shell correlation (3.6 Å at FSC=0.5). C) The local resolution density map of hybrid Aβ42‐αHL (left) and the αHL scaffold at a higher contour (right). The resolution is color‐coded according to the legend on the right. The lower local resolution of the Aβ42 pore region (Low contour, mainly the yellow and red colors), compared to the better local resolution of the αHL part (High contour, mainly the blue and cyan colors) indicates structural flexibility and/or heterogeneity for the pore region. D) Side (upper image) and top (lower image) views of molecular representation of heptameric Aβ42‐αHL in the electron density map (PDB ID: 7O1Q, EMD‐12696). E–G) The comparison of the structures from the side and top views. αHL‐displayed Aβ42 pore (yellow, E); WT αHL transmembrane pore (blue, F) and hexameric Aβ42cc (residues 15–42, light purple, G) built by NMR restrained Rosetta simulation with the smallest pore diameter.[26]