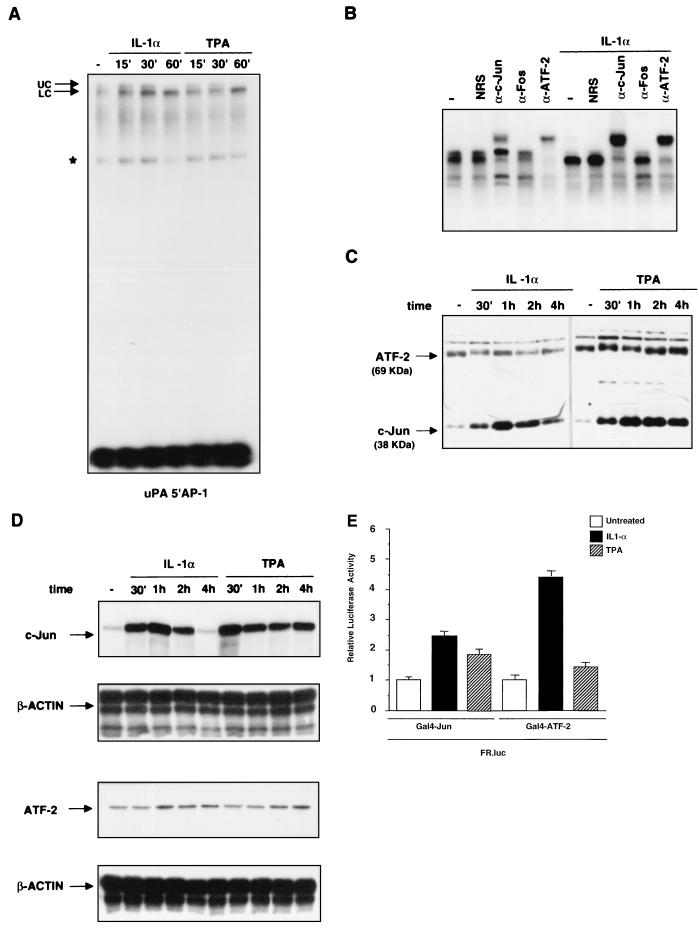
FIG. 6.
In vitro analysis of protein binding to the uPA 5′ AP-1 site during the course of IL-1 or TPA induction. (A) EMSA of IL-1α- and TPA-induced binding to the uPA 5′ AP-1 oligonucleotide. Nuclear proteins were extracted from HepG2 cells at different times (in minutes) after the induction and incubated with the labelled oligonucleotide before the gel retardation assay. (B) Antibody supershift analysis of the complex bound to the uPA 5′ AP-1 oligonucleotide. Nuclear extracts from uninduced or IL-1α- or TPA-induced (60 min) HepG2 cells were preincubated for 3 h with the indicated antibodies, before the addition of the probe (NRS, normal rabbit serum; α-Fos, antibody recognizing all Fos family members). (C) Immunoblotting analysis of ATF-2 and c-Jun expression. Nuclear proteins were extracted at the indicated time points from IL-1α- or TPA-induced cells, separated by SDS-PAGE (20 μg/lane), and transferred to polyvinylidene difluoride membranes. Western blots were first incubated with both anti-c-Jun and anti-ATF-2 antibodies and then with horseradish peroxidase-conjugated secondary antibodies followed by enhanced chemiluminescence. As a control for equal loadings, the blotted proteins were stained with Ponceau red. The arrows indicate the apparent molecular masses of c-Jun (39 kDa) and ATF-2 (69 kDa). (D) RNase protection analysis of c-Jun and ATF-2 mRNA in response to IL-1 induction. Total RNA was extracted at different times (minutes or hours) after induction as indicated and annealed to the human c-Jun or ATF-2 ribonucleotide probes along with the internal reference β-actin ribonucleotide probe, before RNase digestion and polyacrylamide-urea gel electrophoresis. (E) Modulation of c-Jun and ATF-2 transactivation activity by IL-1 and TPA. The FR-luc reporter construct was cotransfected with the pDB10 (containing the mouse c-Jun amino acid residues 1 to 253 fused to the GAL4 DNA-binding domain) or the GAL4-ATF-2 (containing the human ATF-2 amino acid residues 1 to 109 fused to the GAL4 DNA-binding domain) expression vector in the HepG2 cells, which were subsequently treated with IL-1α or TPA. The results represent the averages of three independent experiments.