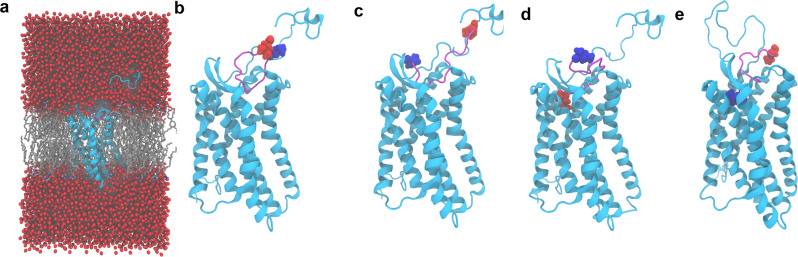
Fig. 1. MD simulations setup and binding modes of the CXCR4/EPI-X4 complex.
CXCR4 (cyan) and EPI-X4 (purple) are shown in cartoon representation and the N-terminal (blue) and C-terminal (red) residues of EPI-X4 are shown as spheres. a Representative all-atom CXCR4/EPI-X4 complex embedded in a POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) lipid bilayer (gray sticks) and water (red spheres) as used in the MD simulations. Bilayer and water are omitted in the other figures of this manuscript for clarity. The initial binding modes represented in (b–d) were obtained using docking calculations and the binding mode shown in (e) was obtained using homology modeling. In (b, c) the middle portion of the peptide is inside the binding pocket (abbreviated as MID-IN1 and MID-IN2, respectively. In d the C-terminus of the peptide is inside the binding pocket (CTER-IN) while in (e), the N-terminus of EPI-X4 is the region inside the binding pocket (NTER-IN).