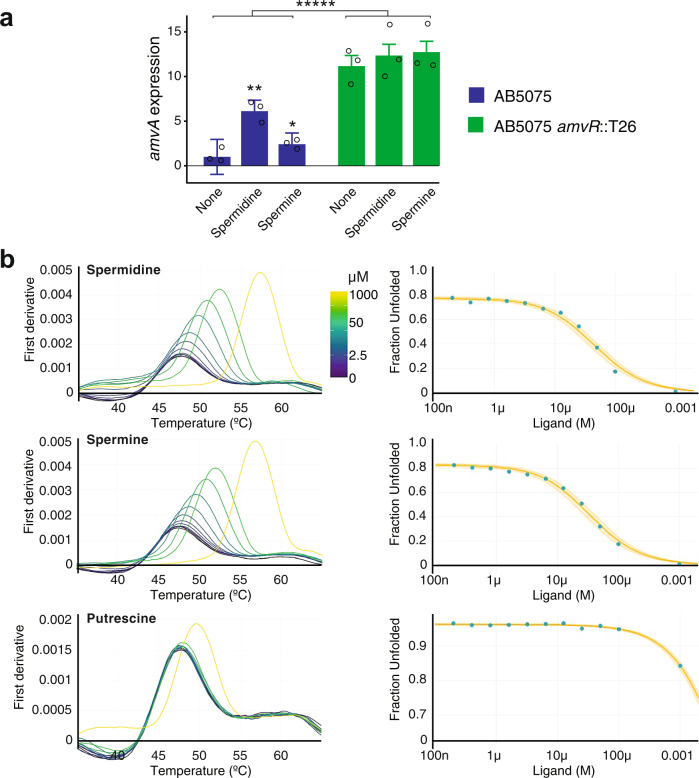
Fig. 4. AmvR and long-chain polyamines control amvA expression.
a AmvR-dependent induction of amvA by long-chain polyamines. Quantitative real-time PCR of amvA transcript in AB5075 and AB5075 amvR::T26 following addition of long-chain polyamines at 1/16 WT MIC (10 mM spermidine, 2 mM spermine). AB5075 amvR::T26 showed dramatically increased amvA transcript levels, which did not increase further on polyamine addition. All fold changes are expressed relative to the untreated AB5075 control. Results shown are the geometric mean ± standard deviation of three biological replicates, which were each comprised of two technical replicates. A two-factor ANOVA showed a significant effect of strain background (*****p < 0.00001) and polyamine treatment (p = 0.0013, comparison not shown) on amvA expression levels. A one-way ANOVA with Dunnett’s post hoc test was used to compare polyamine-treated samples with the untreated sample of the same strain (*p < 0.05, **p < 0.01). b Nano-DSF analysis of purified AmvR shows spermine and spermidine binding at low µM affinity. Melt curves and fitted unfolded protein fraction are shown for AmvR in the presence of increasing polyamine concentrations. Spermidine and spermine prevented protein unfolding at low concentrations (<10 µM), while putrescine only influenced protein unfolding at the highest concentration of 1 mM. The complete experiment was performed in duplicate, and results from the second replicate are provided in Supplementary Fig. 4. Calculated binding affinities are provided in Supplementary Tables 3 and 4.