Figure 1.
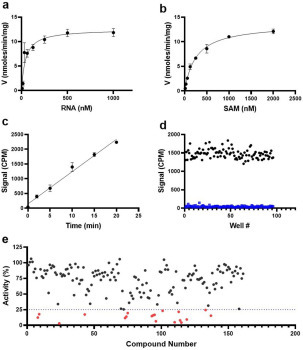
Kinetic characterization and screening of nsp14 MTase activity. The optimized radiometric MTase assay was used to determine the Kmapp and kcatapp values for (a) RNA and (b) SAM at saturating concentrations of SAM and RNA, respectively. (c) The linearity of the initial velocity of the nsp14 MTase reaction was tested at Km for both substrates. (d) The Z′ factor was determined at the same conditions as in c. Nsp14 activity was assessed in the absence (100% activity) ( ) and presence (
) and presence ( ) of 1 µM sinefungin. (e) Nsp14 was screened against a library of SAM analogs. A collection of 161 SAM competitive inhibitors and analogs was tested at 50 µM for inhibition of nsp14 activity under conditions described for Z′-factor determination. Compounds with higher than 75% inhibition (
) of 1 µM sinefungin. (e) Nsp14 was screened against a library of SAM analogs. A collection of 161 SAM competitive inhibitors and analogs was tested at 50 µM for inhibition of nsp14 activity under conditions described for Z′-factor determination. Compounds with higher than 75% inhibition ( ) were selected for follow-up experiments. Values in a, b, and c are presented as the mean ± standard deviation of three independent experiments (n = 3). Values in d and e are from single screening.
) were selected for follow-up experiments. Values in a, b, and c are presented as the mean ± standard deviation of three independent experiments (n = 3). Values in d and e are from single screening.
