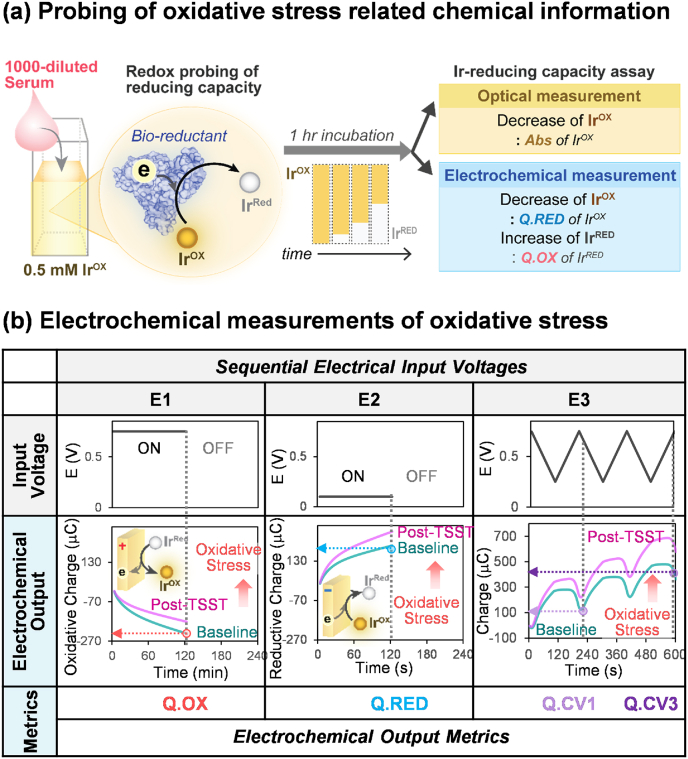
Fig. 2.
Optical and electrochemical metrics of the Ir-reducing capacity assay. (a) A redox-mediator (K2IrCl6, IrOX) is used to detect oxidative stress by probing the reducing capacities of a diluted serum sample. During a 1 h incubation, IrOX is converted to IrRed by accepting electrons from the sample's reductants. Independent measures of the residual IrOX are obtained optically (Abs at 490 nm) and electrochemically (reductive charge; Q.RED), while measures of the generated IrRed are obtained electrochemically (oxidative charge; Q.OX). (b) For the electrochemical measurement, three sequential electrical input voltages are imposed: (i) E1 is a 2-min imposed oxidation voltage (+0.7 V vs Ag/AgCl) that yields one metric (the cumulative oxidative charge during these 2 min; Q.OX); (ii) E2 is a 2-min imposed reduction voltage (+0.1 V vs Ag/AgCl) that yields a second metric (the cumulative reductive charge during these 2 min; Q.RED); and (iii) E3 is a 10-min imposed cyclic voltage (CV) that generates 2 additional metrics (Q.CV1 and Q.CV3) that reflect the dynamic balance between the reduction of the residual IrOX and oxidation of the generated IrRed. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)