Abstract
Diabetes Mellitus (DM) is a metabolic disease characterized by hyperglycemia. Chronic hyperglycemia is associated with long-term dysfunction such as retinopathy, nephropathy, neuropathy and cardiovascular diseases. These complications increase rates of death and disability worldwide. Due to the negative effects of DM on the quality of life, the mechanism and treatments of the disease should be investigated in more detail. Most of the research in diabetes is performed in experimental animals. Experimental animal models contributed to the advancement of clinical research, the development of new therapeutic approaches, the discovery of insulin and the purification of insulin. There are many animal models of DM in the literature. But there are a few DM model studies created with chick embryos. In these studies, it was seen that there were differences in STZ doses and STZ administration techniques. The objective of this study was to create a more acceptable and easier DM model. 180 specific pathogen free (SPF) fertilized chicken eggs (White Leghorn chicken) were used in this study. STZ was administered to 160 SPF eggs for an induced DM model. The remaining 20 SPF eggs were separated as a control group. We used two different DM models (Air sack model (ASM) and Chorioallantoic membrane model (CAMM)) and blood sampling technique in our study. 160 SPF eggs were divided into two groups with 80 eggs in each group, according to the model in which STZ was administered. When the relationship between blood glucose and blood insulin levels were examined, it was determined that there was a significantly strong negative correlation in the control group and ASM 1 group; and a significantly very strong negative correlation was found in the ASM 2 group and ASM 3 group. Our data indicate that the optimal STZ dose to create a DM model was 0.45 mg/egg and the best DM model was ASM. The second technique to be the best blood sampling technique for determining blood glucose levels. We believe that ASM can be used in DM studies and anti-DM drug studies in terms of its easebly, applicability, reproducibility and low cost.
Keywords: Air sack model, Chick embryo, Experimental diabetes model, Streptozotocin
1. Introduction
Diabetes Mellitus (DM) is a metabolic disease characterized by hyperglycemia due to disorder in insulin secretion, insulin sensitivity or both. Chronic hyperglycemia resulting from DM is associated with long-term dysfunction such as retinopathy, nephropathy, neuropathy and cardiovascular diseases (American Diabetes Association, 2010). Diabetic complications due to hyperglycemia have decreased the life quality and expectancy significantly (Sothornwit et al., 2018). DM complications have led to rising rates of death and disability worldwide. According to the Global Disease Burden Study data, DM was reported to be among the top ten causes of reduced human life expectancy (Naghavi et al., 2015). Due to the negative effects of DM on the quality of life, it is concluded that the mechanism of the disease and treatments must be investigated more comprehensively (Couzin-Frankel, 2011).
DM has a complex etiology and mechanisms that affect many systems. Although in vitro and in silico studies to understand the mechanisms of DM are improved, they cannot completely replace the information obtained from animal models (Graham and Schuurman, 2015). Most of the research in diabetes is performed in experimental animals (Gale, 2005, Roep and Atkinson, 2004). Experimental animal models contributed to the advancement of clinical research, the development of new therapeutic approaches, the discovery of insulin and the purification of insulin (Bilous and Donnelly, 2010, Karamitsos, 2011).
There are many animal models of DM in the literature. These models are mainly classified by the type of diabetes as spontaneous or induced (Graham and Schuurman, 2015, King, 2012, Masiello, 2006, Rees and Alcolado, 2005, Srinivasan and Ramarao, 2012). Induced DM models are two types, surgical and non-surgical models. The surgical DM model consists of partial or complete ablation of the pancreas. Non-surgical DM models are performed by administration of toxic substances showing beta cell tropism (i.e. alloxan or streptozotocin), immunosuppressors, viral infectious agents (e.g. Coxsackie B virus) or hypercaloric diets (e.g. high-fat, or high-sucrose) (Furman, 2015, Rees and Alcolado, 2005, Surwit et al., 2013).
Streptozotocin (STZ) and alloxane are commonly used in non-surgical DM models induced by chemical, toxic agent or drug (Rees and Alcolado, 2005). STZ is a potent alkylating agent that has been shown to interfere with glucose transport (Wang and Gleichmann, 1998), glucokinase function (Zähner and Malaisse, 1990) and cause multiple DNA strand breaks (Bolzán and Bianchi, 2002). A single high dose of STZ can produce diabetes in rodents as a result of its toxic effects. Alternatively, small doses of STZ administered consecutively can be used (e. g. 40 mg/kg on five consecutive days). In susceptible rodents, STZ induces insulinopenic diabetes and is involved in immune destruction as in human Type 1 DM. Multiple low-dose STZ models are widely used to study immunological pathways that lead to insulitis and beta cell death (Herold et al., 1997, Holstad and Sandler, 2001, Mensah-Brown et al., 2002, Müller et al., 2002, Yang et al., 2003, Zuccollo et al., 1999).
Legal and ethical restrictions are encountered in studies with experimental animals such as rodents, mammals. Therefore, alternative and new experimental animal models may facilitate the conduct of studies. Among vertebrates, birds are phylogenically closer to mammals. Birds and chicks are preferred models in developmental biology, toxicology, cancer research, immunology and drug testing. Chicken eggs can be easily accessible and they have a short incubation period (Datar and Bhonde, 2011).
In the literature, there are a few DM model studies created with chick embryos. In these studies, it is seen that there are differences in STZ doses and STZ administration techniques (Shi et al., 2014, Sivajothi and Dakappa, 2014, Yoshiyama et al., 2005). Since DM model studies with STZ differed, we aimed to create a more acceptable and easier DM model.
2. Material and methods
This study was performed in Afyonkarahisar Health Sciences University, Medicine Faculty, Department of Anatomy. Ethics committee approval was obtained from Afyon Kocatepe University Animal Ethics Committee (49533702/328).
2.1. Experimental animal and incubation conditions
180 specific pathogen free (SPF) fertilized chicken eggs (White Leghorn chicken) were used in this study. SPF eggs were obtained from Izmir Bornova Veterinary Control Institute (Izmir/Turkey). SPF eggs weighed 65 ± 5 g. SPF eggs were placed in the incubator with sharp ends pointing down to ensure continuity of the embryos. The day of this procedure was accepted as the 0th day of the incubation. SPF eggs were incubated at 37.5 ± 0.2 0C and 65–75% humidity in an incubator that performed automatic cycle every 2 h. The stages of chick embryos were determined according to the Hamburger-Hamilton classification (stage 38 incubation day of 12, stage 44 incubation day of 18) (Hamburger and Hamilton, 1951).
2.2. Chemical and doses
STZ (N-(Methylnitrosocarbamoyl)-α-D-glucosamine, CAS Number: 18883–66-4, Sigma-Aldrich Chemie GmbH, Germany) was dissolved in saline and a stock STZ solution was prepared. STZ doses given to chick embryos were determined according to the literature (Shi et al., 2014, Sivajothi and Dakappa, 2014, Yoshiyama et al., 2005). Four different STZ doses were administered in 50 µl saline solution.
2.3. Experimental groups
On the 12th day of incubation, STZ was administered to 160 SPF eggs for an induced DM model. The remaining 20 SPF eggs were separated as a control group. 160 SPF eggs were divided into two groups (Air sack model (ASM) and Chorioallantoic membrane model (CAMM)), with 80 eggs in each group, according to the model in which STZ was administered.
ASM and CAMM groups were divided into four subgroups, with 20 eggs in each group, according to the STZ doses administered. STZ was administered on the inner shell membrane at doses of 0.15 mg/egg, 0.30 mg/egg, 0.45 mg/egg and 0.60 mg/egg (ASM 1, ASM 2, ASM 3, ASM 4 groups, respectively). STZ was administered in the chorioallantoic membrane at doses of 0.15 mg/egg, 0.30 mg/egg, 0.45 m/egg and 0.60 mg/egg (CAMM 1, CAMM 2, CAMM 3, CAMM 4 groups, respectively). Doses and groups are shown in Table 1.
Table 1.
Groups of diabetes mellitus model.
| Dose Method | STZ (0.15 mg/egg) | STZ (0.30 mg/egg) | STZ (0.45 mg/egg) | STZ (0.60 mg/egg) |
|---|---|---|---|---|
| ASM | Group ASM1 (n = 20) | Group ASM2 (n = 20) | Group ASM3 (n = 20) | Group ASM4 (n = 20) |
| CAMM | Group CAMM1 (n = 20) | Group CAMM2 (n = 20) | Group CAMM3 (n = 20) | Group CAMM4 (n = 20) |
2.4. Diabetes mellitus models
2.4.1. Air sack model
On the 12th day of incubation, after cleaning the eggshell with 70% ethyl alcohol, a small hole was drilled with the tip of extra pointed tweezers on the eggshell. This hole was big enough for the Hamilton injector to pass. Then, four different doses of STZ were injected by Hamilton injector. In order to prevent damage to the inner shell membrane, Hamilton injector was advanced no more than 1 cm from opened hole to air sack (Fig. 1). After STZ injection, hole was closed sterile with parafilm. Eggs were placed in the incubator until 18th day.
Fig. 1.
Air sack model injection technique. (a) Schematic representation, (b) Injection into fertilized egg.
2.4.2. Chorioallantoic membrane model
After cleaning the eggshell with 70% ethyl alcohol, a hole was drilled approximately 2–3 cm2 on the eggshell with extra pointed tweezers. The surface of the inner shell membrane was seen through opened hole. Then the inner shell membrane was opened about 2 cm2 with extra pointed tweezers. After this stage, four different doses of STZ were injected by Hamilton injector into the chorioallantoic membrane (Fig. 2). No damage was done to any vessel and embryo during all procedures. After STZ injection, hole was closed with sterile parafilm. Eggs were placed in the incubator until 18th day.
Fig. 2.
Chorioallantoic membrane model injection technique. (a) Schematic representation, (b) Injection into fertilized egg.
2.5. Blood sampling techniques
Experimental groups and control were opened to measure blood glucose and blood insulin levels on the 18th day of incubation. Blood samples from SPF eggs were made using two different techniques. Accordingly, if blood was taken with the first technique, it was defined as “a”, if blood was taken with the second technique, it was defined as “b”.
2.5.1. First technique (“a”)
All SPF eggs were examined under the light source for determining the air sack area. Then the eggshell above the air sack was sterilized with 70% ethyl alcohol, it was carefully opened with curved tip forceps. The ear stick was moistened with distilled water. The moist ear stick was moved over the inner shell membrane with gentle movements. The white membrane (inner shell membrane), which does not show the structures under it, was made transparent with a moist ear stick. Thus, the blood vessels became visible under the inner shell membrane. Then, the inner shell membrane was pulled in the same direction as the vessel with the dry ear stick. Vessel was brought closer to the inner shell membrane. Blood was collected from this vessel with a 30GX13 mm mesotherapy needle attached to the tip of the 1 ml disposable insulin syringe. At least 200 µl of blood samples were taken from the chick embryos with this technique (Fig. 3).
Fig. 3.
Blood sampling with the first technique. (a) Transparency with a moist ear stick, (b) Entering the visible vessel to take blood, (c) Taking a blood sample, (d) Blood glucose measurement.
2.5.2. Second technique (“b”):
After removing the egg shell with curved tip forceps, the inner membrane shell was carefully removed with extra pointed tweezers. Then the embryo was placed on a sterile plate. The amniotic membrane around the embryo was quickly removed with extra pointed tweezers. Chick embryo was placed in the supine position, the sternum and ribs were cut with sterile scissors. The heart surrounded by a pericardium was seen in the thoracic cavity. The pericardium was carefully dissected with extra pointed tweezers. All chick embryos' hearts were beating. Then, with a 30GX13 mm mesotherapy needle attached to the tip of the 1 ml disposable insulin syringe blood was tried to aspirate from the heart. During the embryo being taken into the sterile plate, the integrity of the vascular plexus around the embryo was disrupted. As the amount of intravascular blood decreased due to bleeding, the amount of blood in the heart was not sufficient to measure the insulin levels (Fig. 4). Therefore, in our study with this technique, we could not measure insulin levels. Afterwards, the heart was dissected and the blood glucose level was measured directly from the heart with a strip attached to the blood glucose meter. Devices, strip and kit are shown in Table 2.
Fig. 4.
Blood sampling with the second technique. (a) Opening the chest wall, (b) Observing the heart in the chest cavity, (c) Taking a blood sample from the heart, (d) Dissecting the heart, (e) Placing the blood strip in the heart, (f) Blood glucose measurement.
Table 2.
Material list.
| Blood Parameters | Strip/Kit | Device |
|---|---|---|
| Blood Glucose | Accu Chek Performa Nano Blood Strip (Roche, Basel, Switzerland) | Accu Chek Performa Nano Glucometer (Roche, Basel, Switzerland) |
| Blood Insulin | Chicken Insulin ELISA Kit E0048Ch (BT-Lab, Shanghai, China) | ChemWell 2910 Automated PC Controlled ELISA/Biochemistry Analyzer (Awareness Technology, Palm City, USA) |
2.6. Statistical analysis
Statistical analysis of the data was performed with the IBM Statistical Package for the Social Sciences (SPSS 24.0) program. Kolmogorov Smirnov test was used to determine the normal distribution of data. Kruskal-Wallis test was used to compare the groups since the data were not normally distributed and n ≤ 30. Dunn test were employed as post-hoc tests and p < 0.05 were considered significant. Pearson's correlation analysis was used to determine whether there is a linear relationship between blood glucose and blood insulin measurements, and if so, the direction and severity of this relationship. Mean statistical values were expressed as Mean ± Standard Deviation (Mean ± SD).
3. Results
We used two different DM models (ASM and CAMM) and blood sampling techniques in our study. It was determined that all chick embryos in CAMM (Fig. 5) and ASM 4 (Fig. 6) groups died on the 18th day of incubation. In ASM groups, the blood insulin level could not be measured because sufficient blood samples could not be taken from the heart with the second technique. Statistical data on blood glucose and blood insulin levels of ASM groups were presented in Table 3.
Fig. 5.
Dead embryos in chorioallantoic membrane model. (a) Opening the eggshell, (b) Dead embryo.
Fig. 6.
Dead embryos in air sack model. (a) Opening the eggshell, (b) Dead embryo.
Table 3.
According to techniques blood glucose and blood insulin levels.
| Groups | Blood Glucose (mg/dL) Mean ± SD |
Blood Insulin (mIU/L) Mean ± SD |
||
|---|---|---|---|---|
| Blood Sampling Technique |
Blood Sampling Technique |
|||
| First Technique | Second Technique | First Technique | Second Technique | |
| Control 18th day | 124.70 ± 12.3 | 139.60 ± 9.96 | 6.08 ± 2.18 | – |
| ASM 1 | 132.90 ± 8.67 | 154.60 ± 8.95 | 4.34 ± 1.49 | – |
| ASM 2 | 137.50 ± 10.11 | 185.40 ± 12.53a,b | 3.57 ± 0.90 | – |
| ASM 3 | 139.90 ± 6.62a | 189.20 ± 19.66a,b | 3.28 ± 1.22a | – |
There was a statistically significant difference with the control group. p < 0.05, Kruskal-Wallis test, Dunn test as post-hoc test
There was a statistically significant difference with ASM 1 group. p < 0.05, Kruskal-Wallis test, Dunn test as post-hoc test
Blood glucose levels were compared according to the first technique. A statistically significant difference was found only between the control group and the ASM 3 group (p = 0.019). When the blood glucose levels were compared according to the second technique, statistically significant differences were found between the control group with ASM 2 and ASM 3 groups (p < 0.001, p < 0.001, respectively). There were also differences between ASM 1 with ASM 2 and 3 groups (p = 0.029, p = 0.014, respectively). However, according to both blood sampling techniques, mean blood glucose levels were increasing from the control group to the ASM 3 group.
Blood insulin level could not be measured with the second technique. When the blood insulin levels were examined with the first technique, it was determined that there was a decrease from the control group to the ASM 3 group. A statistically significant difference was found only between the control group and the ASM 3 group (p = 0.018).
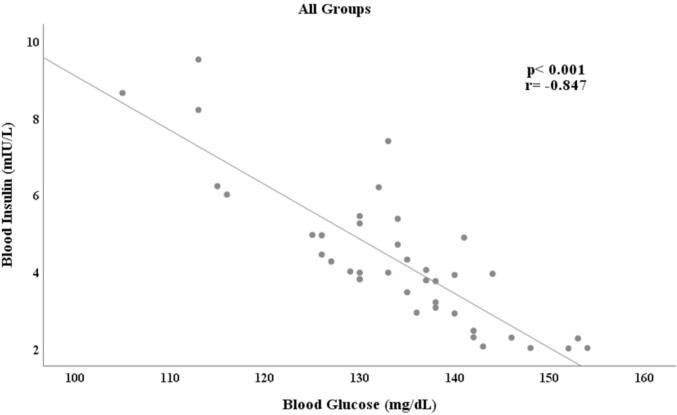
In Table 3, blood glucose and insulin levels were evaluated according to the first technique. Accordingly, a decrease in blood insulin levels and an increase in blood glucose levels were determined depending on the STZ dose (Fig. 7).
Fig. 7.
Correlation analysis of all blood glucose and blood insulin levels measured in the first technique.
When the relationship between blood glucose and blood insulin levels were examined, it was determined that there was a significantly strong negative correlation in the control group and ASM 1 group; and a significantly very strong negative correlation was found in the ASM 2 group and ASM 3 group. Correlation coefficients and p values were presented in Fig. 8.
Fig. 8.
Correlation analysis between blood glucose and insulin levels by groups.
4. Discussion
In this study, we presented a new experimental STZ induced DM model in chick embryos. In the literature, there are a few STZ induced DM model in chick embryos. When looking at these studies, there are differences in STZ doses and STZ administration techniques (Shi et al., 2014, Sivajothi and Dakappa, 2014, Yoshiyama et al., 2005). Therefore, we aimed to find the optimal STZ application location and the most appropriate STZ dose to create DM in chick embryos.
As far as we investigated, STZ were used on 14th day in two studies(Sivajothi and Dakappa, 2014, Yoshiyama et al., 2005) and STZ and glucose were used on 12th day in other one study(Shi et al., 2014) in chick embryos. In our study, different doses of STZ were applied on the 12th day of incubation to create a DM model. Aim of using STZ on 12th day of incubation is development of the pancreas started on the 5th day and completed on the 12th day. Insulin production starts at day 5 but increases until day 12. In addition, serum insulin levels could not be measured until day 12 of chick embryo development (Haselgrübler et al., 2017, Sivajothi and Dakappa, 2014, Yoshiyama et al., 2005).
In experimental studies, the route of administration is as important as the day the substance is given. While Yoshiyama et al. and Sivajothi et al. injected STZ into the albumen (Sivajothi and Dakappa, 2014, Yoshiyama et al., 2005), Shi et al. injected in to amnion layer of each egg (Shi et al., 2014). Differences in the administration of STZ (Shi et al., 2014, Sivajothi and Dakappa, 2014, Yoshiyama et al., 2005), we can attribute the transition time of STZ to the embryonic circulation and the possible toxic effect accordingly, as well as the damage that may occur in the embryo depending on the area of application. For the reasons we mentioned above, we applied STZ on the inner shell membrane and CAM, not direct albumen or amnion layer.
In our study, we determined that in the CAMM, all embryos died on the 18th day of incubation. In another study that made an application to CAM, it was reported that the embryos were alive (Haselgrübler et al., 2017). In our study, while embryos were examined 6 days after the injection (18th of incubation) they were examined at different intervals (up to 3 h, 11th of incubation) in the literature (Haselgrübler et al., 2017). CAMM is generally used to create cancer in chick embryos. As early as 1913, scientists discovered that tumor grafts can be cultivated by the rich capillary plexus of the CAM surrounding the chick embryo. The CAM model was developed as a model of angiogenesis in the 1970 s, and by the 1980 s, it was identified as a tool to study tumor metastasis (Auerbach et al., 1975, Liu et al., 2013, Murphy, 1913, Ossowski and Reich, 1980). We think that due to the high vascularization in CAM, STZ rapidly enters the embryonic circulation, shows acute toxicity and kills chick embryos in our study. We believe that in ASM, the embryo is protected from the toxic effects of STZ because the STZ is slowly absorbed through the inner shell membrane.
We conducted a literature search for STZ injection doses applied to induce diabetes in chick embryos. Yoshiyama et al. aimed to create DM model with three different doses of STZ in their study (0.1, 0.3 and 1 mg/egg). In their study, they stated that 0.3 mg/egg STZ dose is the ideal dose for increasing blood glucose and decreasing serum insulin levels. However, they had accepted 1 mg/egg STZ dose as overdose (Yoshiyama et al., 2005). Sivajothi et al. had created DM model with 0.3 mg/egg STZ dose, similar to the study of Yoshiyama et al. (Sivajothi and Dakappa, 2014). Apart from that, in the study of Shi et al, it was determined that three different STZ doses varying between 250 and 300 mg/kg-egg were used (Shi et al., 2014). In line with this information, DM model was tried to be created with four different STZ doses in our study. STZ doses were injected on the inner shell membrane and CAM. Due to the toxicity of STZ, all embryos in the CAMM groups and ASM 4 group died. Considering the survival rates of the embryos and the state of DM formation, we determined that the injection of STZ doses on the inner shell membrane gave best results. As seen in Table 3, when blood glucose and blood insulin levels were evaluated together, a statistically significant difference was found between the 0.45 mg/egg STZ group and the control group. According to this result, 0.45 mg/egg STZ dose was determined to be the optimal/ideal dose for the DM model in our study. There was no statistical difference among the results of 0.15, 0.30 mg/egg STZ doses and the control group in terms of creating DM. However, it was determined that 0.6 mg/egg STZ dose kills embryos due to its toxic effect. In this context, the dose of 0.6 mg/egg STZ was determined as the lethal dose.
In literature, it was determined that different incubation days and blood sampling techniques were used (Shi et al., 2014, Sivajothi and Dakappa, 2014, Yoshiyama et al., 2005). Yoshiyama et al. and Sivajothi et al. selected large vitelline vessels under the fluorescent lamp and marked by pencil. After that an artery blood was collected by means of tuberculin syringe on the 17th day of incubation (Sivajothi and Dakappa, 2014, Yoshiyama et al., 2005). Shi et al. collected blood from chick hearts for blood glucose and insulin levels on 18th day of incubation. For insulin detection, they sampled 200–300 µl blood from chick embryo’s heart (Shi et al., 2014). In our study, blood samples were taken by two different blood sampling techniques on the 18th day of incubation to measure blood glucose and insulin levels. We made the vessel visible under the inner shell membrane by moistening the ear stick with saline and moving it over the inner shell membrane in the first technique. With this blood collection technique, we were easily able to sample enough blood to measure glucose and insulin levels. In the second technique, we tried to sample blood from heart, but we could not collect enough blood for measuring glucose and insulin levels because of bleeding that as a result of disrupting vascular plexus. So, blood glucose level was measured directly from blood in the heart with the strip attached to the blood glucometer.
In literature, there are few data about glucose and insulin levels in chick embryos. On the 18th day of incubation, Vladimirov et al. and Shi et al. determined blood glucose levels between 144 and 150 mg/dL, 150 and 160 mg/dL, respectively (Shi et al., 2014, Vladimirov, 1931). Thommes et al. reported that the mean glucose level was 227.9 ± 16.7 mg/dL on the 18th day (Thommes and Tambornino, 1962). In the study of Lu et al., they found that the blood glucose levels of the 18th day of incubation varied between 190 and 215 mg/dL (Lu et al., 2007). Yoshiyama et al. determined mean blood glucose level 157 ± 15 mg/dL on the 17th incubation day. Yoshiyama et al. determined mean insulin level 143 ± 18 pg/mL on the 17th incubation day (Yoshiyama et al., 2005). On the 18th day of incubation, while Lu et al. found insulin levels between 300 and 400 pg/mL (Lu et al., 2007), Shi et al. detected between 7 and 8 µIU/mL (Shi et al., 2014). In the literature, there is no specific reference range to be considered as a control group due to variations in blood glucose and insulin levels. These variations may be due to different incubation days, different blood sample techniques or blood taken from different regions. Considering these variations, pilot study (unpublished manuscript) was conducted and different blood sampling techniques were tried. As a result of the pilot study (unpublished manuscript), two of the blood sampling techniques (first and second technique) were preferred in this study because they were easier and more applicable.
In our STZ-induced DM model study, blood glucose and insulin levels were measured in control group on the 18th day of incubation. The blood glucose and insulin levels of control group were compared with experimental groups. It was determined that there was an increase in blood glucose levels of both blood sampling techniques depending on the STZ dose. It was also found that there was a decrease in blood insulin levels inversely proportional to the increase in blood glucose levels. (Blood insulin level was measured with only first blood sampling technique). Mean blood glucose and insulin levels are shown in Table 3When the results are analyzed, we think that the reason for the differences of the glucose levels between the two techniques are that blood is taken directly from the heart in second technique and from the peripheral vessels in first technique.
In the literature, it has been reported that blood glucose levels differ according to the vessels from which blood is taken, the location of the vessel, and the conditions from which the blood is taken, both in clinical studies and in experimental animal studies (Bergman et al., 1970, Coulic et al., 2018, Hof, 1956). In addition, in the experimental study conducted in rats, it has been shown that the heart blood glucose level is the same or higher than the peripheral (Coulic et al., 2018). The reason for the difference between peripheral and heart glucose levels is that glucose is stored in the liver, neo genesis and liver blood outflow reaches directly the heart (Adeva-Andany et al., 2016, Farmer et al., 2008, Matsuda and Brennan, 2012, Sparacino et al., 2012).
5. Conclusion
In our study, we created a new induced DM model by injecting STZ on the inner shell membrane of chick embryos. According to the results of this study, we determined that the optimal STZ dose to create a DM model was 0.45 mg/egg and the best DM model was ASM. We consider the second technique to be the best blood sampling technique for determining blood glucose levels. However, we report that it would be more accurate to measure the blood glucose level in the blood taken directly from the heart rather than the blood taken from the peripheral vessel. Nevertheless, it should not be ignored that there may be differences in glucose level in clinical practice and these may be affected by metabolic-pathological processes.
In conclusion, we believe that ASM can be used in DM studies and anti-DM drug studies in terms of its easebly, applicability, reproducibility and low cost.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Prof. Dr. Tolga Ertekin, for their expert technical assistance.
Data sharing
Data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Erhan Bozkurt, Email: drerhanbozkurt@gmail.com.
Emre Atay, Email: eemreatay@gmail.com.
Abdülkadir Bilir, Email: fztabdulkadirbilir@gmail.com.
Ayşe Ertekin, Email: doktorayse6@yahoo.com.
Halit Buğra Koca, Email: bugrakoca@yahoo.com.
Mehmet Cem Sabaner, Email: drmcemsabaner@yahoo.com.
References
- Adeva-Andany M.M., Pérez-Felpete N., Fernández-Fernández C., Donapetry-García C., Pazos-García C. Liver glucose metabolism in humans. Biosci. Rep. 2016;36 doi: 10.1042/BSR20160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:62–69. doi: 10.2337/dc10-S062. [DOI] [Google Scholar]
- Auerbach, R., Arensman, R., Kubai, L., Folkman, J., 975. Tumor-induced angiogenesis: lack of inhibition by irradiation. Int J Cancer 15, 241–245. [DOI] [PubMed]
- Bergman, E.N., Katz, M.L., Kaufmann, C.F., 1970. Quantitative aspects of hepatic and portal glucose metabolism and turnover in sheep. Am J Physiol 219, 785–793. [DOI] [PubMed]
- Bilous R., Donnelly R. Handbook of Diabetes. John Wiley & Sons; 2010. Part 1. Introduction oto diabetes. Chapter 2. History of diabetes. [DOI] [Google Scholar]
- Bolzán A.D., Bianchi M.S. Genotoxicity of Streptozotocin. Mutat. Res. - Rev. Mutat. Res. 2002;512(2-3):121–134. doi: 10.1016/S1383-5742(02)00044-3. [DOI] [PubMed] [Google Scholar]
- Coulic, V., Dobos, S., Novikov, V., 2018. Glucose measure in arterial or in venous blood. Endocrinol. Int. J. 6, 336–338. https://doi.org/10.15406/emij.2018.06.00199
- Couzin-Frankel J. Trying to reset the clock on type 1 diabetes. Science (80-. 2011;333(6044):819–821. doi: 10.1126/science:333.6044.819. [DOI] [PubMed] [Google Scholar]
- Datar S.P., Bhonde R.R. Modeling chick to assess diabetes pathogenesis and treatment. Rev. Diabet. Stud. 2011;8(2):245–253. doi: 10.1900/RDS.2011.8.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer T.G., Edgar T.F., Peppas N.A. The future of open- and closed-loop insulin delivery systems. J. Pharm. Pharmacol. 2008;60:1–13. doi: 10.1211/jpp.60.1.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015;70:5–47. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- Gale E.A.M. Do dogs develop autoimmune diabetes? Diabetologia. 2005;48(10):1945–1947. doi: 10.1007/s00125-005-1924-y. [DOI] [PubMed] [Google Scholar]
- Graham M.L., Schuurman H.J. Validity of animal models of type 1 diabetes, and strategies to enhance their utility in translational research. Eur. J. Pharmacol. 2015;759:221–230. doi: 10.1016/j.ejphar.2015.02.054. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88(1):49–92. doi: 10.1002/(ISSN)1097-468710.1002/jmor.v88:110.1002/jmor.1050880104. [DOI] [PubMed] [Google Scholar]
- Haselgrübler R., Stübl F., Essl K., Iken M., Schröder K., Weghuber J., Kanzaki M. Gluc-HET, a complementary chick embryo model for the characterization of antidiabetic compounds. PLoS One. 2017;12(8):e0182788. doi: 10.1371/journal.pone.0182788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold K.C., Lenschow D.J., Bluestone J.A. CD28 / B7 Regulation of Autoimmune. Diabetes. 1997;16(1):71–84. doi: 10.1007/BF02786324. [DOI] [PubMed] [Google Scholar]
- Hof J. Comparative studies on protein and blood sugar content of blood in heart and hepatic vein. Arztl Forschr. 1956;10:9–11. [PubMed] [Google Scholar]
- Holstad M., Sandler S. A transcriptional inhibitor of TNF-α prevents diabetes induced by multiple low-dose streptozotocin injections in mice. J. Autoimmun. 2001;16(4):441–447. doi: 10.1006/jaut.2001.0506. [DOI] [PubMed] [Google Scholar]
- Karamitsos D.T. The story of insulin discovery. Diabetes Res. Clin. Pract. 2011;93:2–8. doi: 10.1016/S0168-8227(11)70007-9. [DOI] [PubMed] [Google Scholar]
- King A.J.F. The use of animal models in diabetes research. Br. J. Pharmacol. 2012;166:877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Scanlon C.S., Banerjee R., Russo N., Inglehart R.C., Willis A.L., Weiss S.J., D'Silva N.J. The histone methyltransferase EZH2 mediates tumor progression on the chick chorioallantoic membrane assay, a novel model of head and neck squamous cell carcinoma. Transl. Oncol. 2013;6(3):273–281. doi: 10.1593/tlo.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.W., McMurtry J.P., Coon C.N. Developmental changes of plasma insulin, glucagon, insulin-like growth factors, thyroid hormones, and glucose concentrations in chick embryos and hatched chicks. Poult. Sci. 2007;86(4):673–683. doi: 10.1093/ps/86.4.673. [DOI] [PubMed] [Google Scholar]
- Masiello P. Animal models of type 2 diabetes with reduced pancreatic β-cell mass. Int. J. Biochem. Cell Biol. 2006;38(5-6):873–893. doi: 10.1016/j.biocel.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Matsuda E., Brennan P. The effectiveness of continuous subcutaneous insulin pumps with continuous glucose monitoring in outpatient adolescents with type 1 diabetes: A systematic review. JBI Libr. Syst. Rev. 2012;10:1–10. doi: 10.11124/jbisrir-2012-170. [DOI] [PubMed] [Google Scholar]
- Mensah-Brown E.P.K., Stosic Grujicic S., Maksimovic D., Jasima A., Shahin A., Lukic M.L. Downregulation of apoptosis in the target tissue prevents low-dose streptozotocin-induced autoimmune diabetes. Mol. Immunol. 2002;38(12-13):941–946. doi: 10.1016/S0161-5890(02)00021-4. [DOI] [PubMed] [Google Scholar]
- Müller A., Schott-Ohly P., Dohle C., Gleichmann H. Differential regulation of Th1-type and Th2-type cytokine profiles in pancreatic islets of C57BL/6 and BALB/c mice by multiple low doses of streptozotocin. Immunobiology. 2002;205:35–50. doi: 10.1078/0171-2985-00109. [DOI] [PubMed] [Google Scholar]
- Murphy J.B. Transplantability of tissues to the embryo of foreign species: Its bearing on questions of tissue specificity and tumor immunity. J. Exp. Med. 1913;17:482–493. doi: 10.1084/jem.17.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi M., Wang H., Lozano R., Davis A., Liang X., Zhou M. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Reich E. Experimental model for quantitative study of metastasis. Cancer Res. 1980;40:2300–2309. [PubMed] [Google Scholar]
- Rees D.A., Alcolado J.C. Animal models of diabetes mellitus. Diabet. Med. 2005;22:359–370. doi: 10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- Roep B.O., Atkinson M. Animal models have little to teach us about Type 1 diabetes: 1. support of this proposal. Diabetologia. 2004;47:1650–1656. doi: 10.1007/s00125-004-1517-1. [DOI] [PubMed] [Google Scholar]
- Shi L., Ko M.L., Huang C.-Y., Park S.-Y., Hong M.-P., Wu C., Ko G.-P. Chicken Embryos as a Potential New Model for Early Onset Type i Diabetes. J. Diabetes Res. 2014;2014:1–10. doi: 10.1155/2014/354094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivajothi, V., Dakappa, S.S., 2014. In vitro and in silico antidiabetic activity of pyran ester derivative isolated from Tragia cannabina. Asian Pac. J. Trop. Biomed. 4, 455–459. https://doi.org/10.12980/APJTB.4.2014C1049 [DOI] [PMC free article] [PubMed]
- Sothornwit J., Srisawasdi G., Suwannakin A., Sriwijitkamol A. Decreased health-related quality of life in patients with diabetic foot problems. Diabetes. Metab. Syndr. Obes. Targets Ther. 2018;11:35–43. doi: 10.2147/DMSO.S154304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparacino G., Zanon M., Facchinetti A., Zecchin C., Maran A., Cobelli C. Italian contributions to the development of continuous glucose monitoring sensors for diabetes management. Sensors (Switzerland) 2012;12:13753–13780. doi: 10.3390/s121013753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K., Ramarao P. Animal models in type 2 diabetes research: An overview K. Indian J. Med. Res. 2012;125:451–472. [PubMed] [Google Scholar]
- Surwit R.S., Kuhn C.M., Cochrane C., McCubbin J.A., Feinglos M.N. Synthesis of hollow polymer microspheres. J. Tianjin Polytech. Univ. 2013;32:48–51. [Google Scholar]
- Thommes R.C., Tambornino A. Effects of insulin administration upon blood-glucose levels of the chick embryo. Physiol. Zool. 1962;35(3):256–262. [Google Scholar]
- Vladimirov G. The effect of some factors upon the blood sugar of embryo chicks. J Physiol. 1931;72:411–424. doi: 10.1113/jphysiol.1931.sp002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Gleichmann H. GLUT2 in pancreatic islets: crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes. 1998;47(1):50–56. doi: 10.2337/diabetes.47.1.50. [DOI] [PubMed] [Google Scholar]
- Yang Z., Chen M., Fialkow L.B., Ellett J.D., Wu R., Nadler J.L. The novel anti-inflammatory compound, lisofylline, prevents diabetes in multiple low-dose streptozotocin-treated mice. Pancreas. 2003;26(4):e99–e104. doi: 10.1097/00006676-200305000-00021. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y., Sugiyama T., Kanke M. Experimental diabetes model in chick embryos treated with streptozotocin. Biol. Pharm. Bull. 2005;28(10):1986–1988. doi: 10.1248/bpb.28.1986. [DOI] [PubMed] [Google Scholar]
- Zähner D., Malaisse W.J. Kinetic behaviour of liver glucokinase in diabetes. I. Alteration in streptozotocin-diabetic rats. Diabetes Res. (Edinburgh. 1990;Lothian) 14:101–108. [PubMed] [Google Scholar]
- Zuccollo A., Navarro M., Frontera M., Cueva F., Carattino M., Catanzaro O.L. The involvement of kallikrein-kinin system in diabetes type I (insulitis) Immunopharmacology. 1999;45(1-3):69–74. doi: 10.1016/S0162-3109(99)00149-6. [DOI] [PubMed] [Google Scholar]