Figure 2.
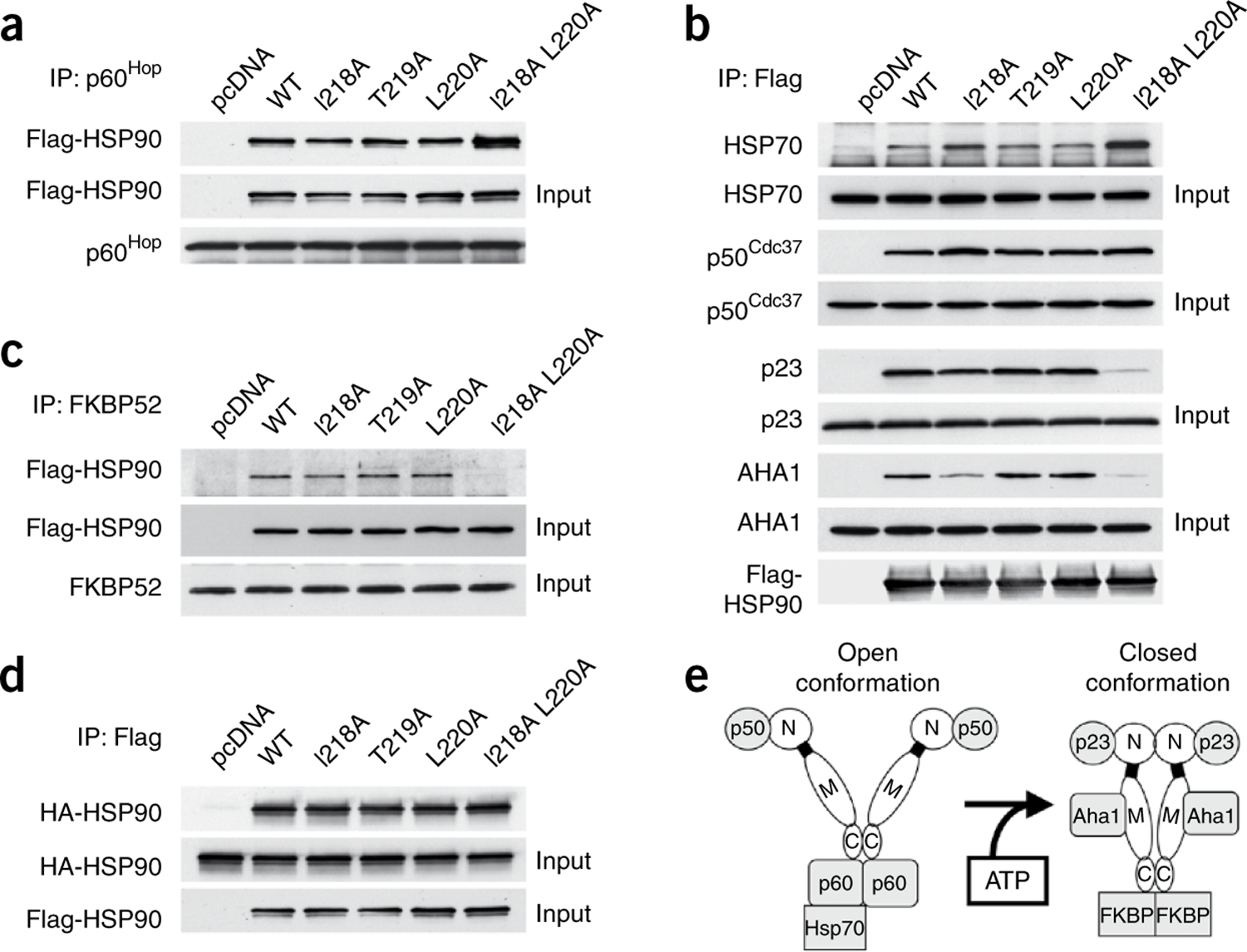
Mutation of β-strand 8 attenuates co-chaperone interaction. (a–d) COS7 cells were transfected with empty vector (pcDNA) or the indicated Flag-HSP90 constructs. Cells were lysed and proteins were immunoprecipitated (IP) by p60Hop antibody (a), by Flag antibody–conjugated agarose (b,d) or by FKBP52 antibody (c) and then separated by SDS-PAGE. Indicated co-precipitating proteins were detected by immunoblotting. WT, wild type. (e) Schematic model for open and closed HSP90 conformation. HSP90 (N, N domain; bold line, charged linker; M, middle domain; C, C domain) binds to p60Hop (p60), HSP70 and p50Cdc37 (p50) in open conformation, and binds to FKBP52 (FKBP), p23 and AHA1 in closed conformation.
