Figure 4.
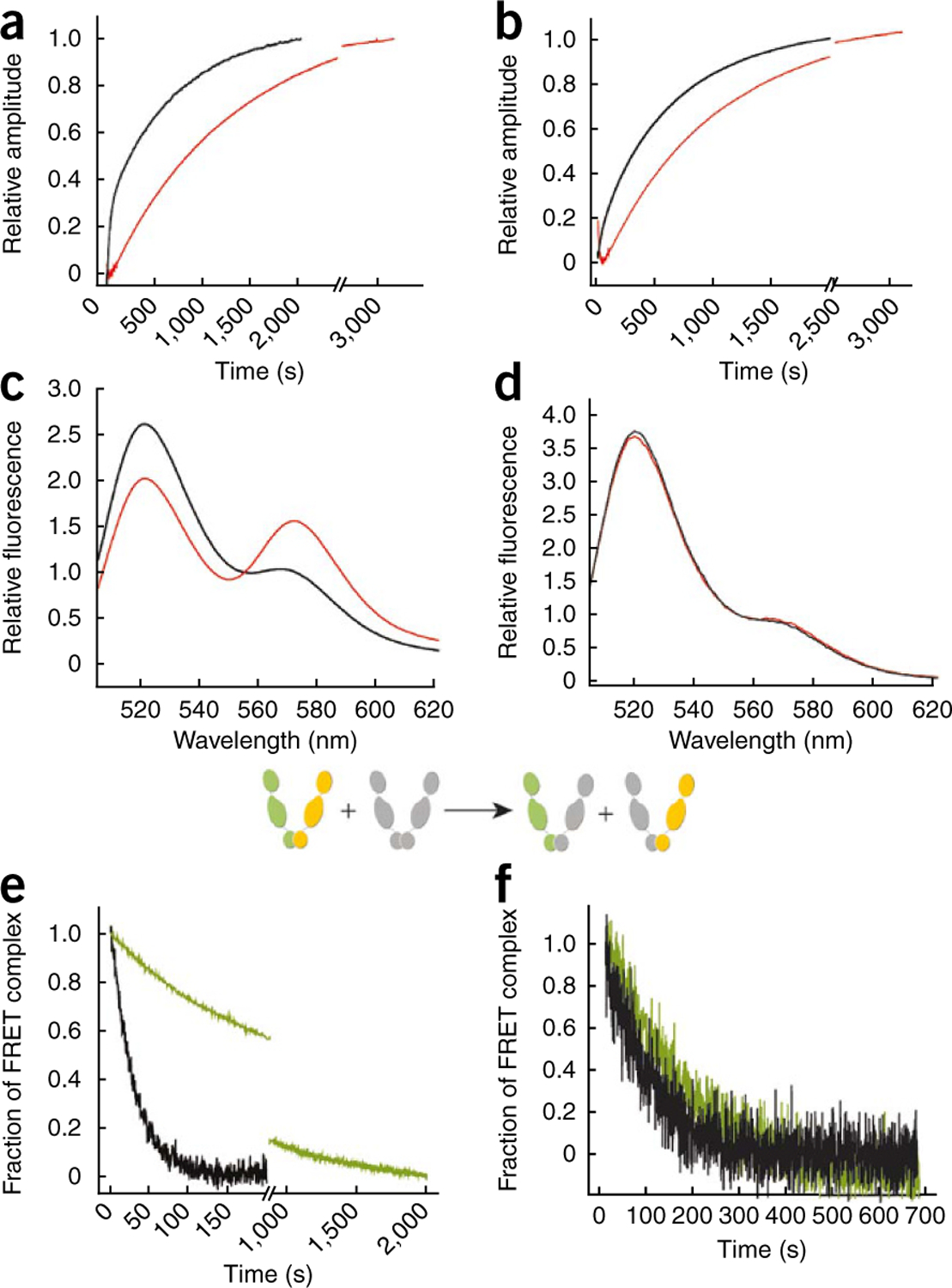
Mutation of β-strand 8 inhibits ATP-induced Hsp90 conformational dynamics. ATPγS-induced kinetics of conformational changes in Hsp90 were measured by FRET analysis after rapid mixing. (a) 200 nM wild-type yeast Hsp90 (black traces) or yeast Hsp90 I205A (red traces) proteins were labeled with the fluorescent dyes ATTO488 (donor) and ATTO550 (acceptor) at the S61C substitution in the N domain of the respective protomers6. (b) Hsp90 proteins were labeled at S61C in the N domain of one protomer and at Q385C in the M domain of the second protomer6. (c) Aha1-induced conformational changes in wild-type yeast Hsp90 were assessed by monitoring FRET efficiencies of Hsp90 S61C labeled with ATTO488 and ATTO550 in the absence (black trace) or in the presence (red trace) of 10 µM Aha1. (d) The same experiment as in c was performed using yeast Hsp90 S61C I205A. (e) Aha1-dependent stabilization of the FRET complex between protomers of wild-type Hsp90 was assayed by a chase with unlabeled wild-type Hsp90. This leads to a dissociation of donor- and acceptor-labeled complexes and a decrease in FRET signal (see scheme insert). In the presence of 10 µM Aha1, the FRET complex is more resistant to dissociation (green trace) than it is in the absence of Aha1 (black trace). (f) The same experiment as in e but using Hsp90 I205A protein. In this case, Aha1 is unable to stabilize the FRET complex.
