Figure 5.
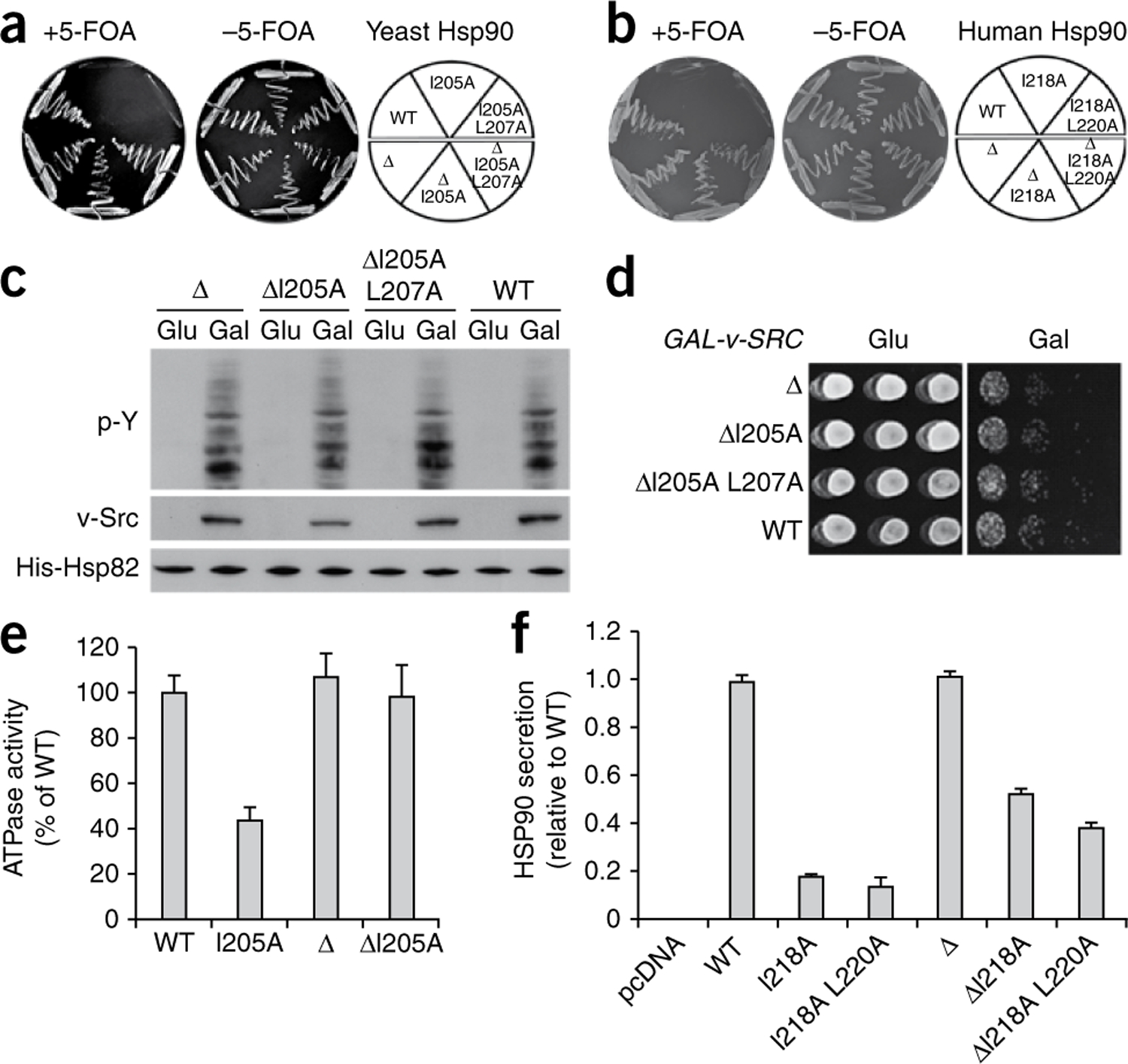
Truncation of the charged linker restores function to β-strand 8 Hsp90 mutants. (a,b) An S. cerevisiae strain with both genomic copies of yeast HSP90 deleted but carrying both wild-type yeast HSP82 (on a URA3-marked plasmid) and either yeast Hsp90 constructs (∆: ∆214–261; a) or human Hsp90α constructs (∆: ∆227–281; b). Cells were grown on SD –TRP plates either with or without 5-FOA at 25 °C for 5 d. (c,d) v-Src under control of the GAL1 promoter was transformed into the same cells expressing the charged linker–deleted wild-type (∆) and mutant versions, ∆I205A and ∆I205A L207A. Growth of these cells in the presence of glucose (Glu) represses v-Src expression, which is induced with galactose (Gal). In vitro v-Src activity and protein expression were detected by immunoblotting (c), and in vivo v-Src activity was determined by its effects on cell growth (d), by immunoblotting using antibodies to phosphotyrosine (p-Y) and v-Src, respectively. (e) ATPase activities of wild-type and indicated mutant Hsp82 proteins. Data are displayed as mean ± s.d. (f) COS7 cells were transfected with empty vector (pcDNA) or indicated Flag-Hsp90 constructs (∆: ∆227–281). After incubation, conditioned medium was collected and secreted Hsp90 protein was detected by ELISA. Data are displayed as mean ± s.d.
