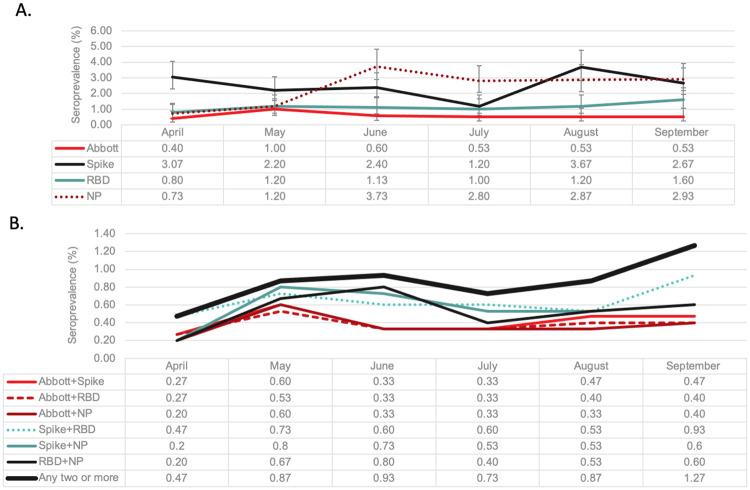
Fig 1.
A. Seroprevalence by month over the first COVID-19 wave in Canada by individual assays. Each line represents seroprevalence rates (summarized in table below) monthly between April and September 2020 (during the first COVID-19 Wave) based thresholds for each assay. Abbott Architect SARS-Cov-2 IgG assay (Abbott-NP) and three in-house IgG ELISA assays recognizing distinct recombinant viral antigens: full length spike glycoprotein (Spike), spike glycoprotein receptor binding domain (RBD), and nucleocapsid (NP). B. Seroprevalence by month over the first COVID-19 wave in Canada by various composite reference standards (results from four anti-SARS-CoV-2 immunoassays). Each line represents seroprevalence rates based on predefined definitions. CRS based on a combination of reactive samples using Abbott-NP, Spike, RBD and NP. Positivity based on “any two or more” was determined by a reactive sample from two or more assays. Since we are not comparing CRS, we did not include 95% CI for each data point (all overlapping).