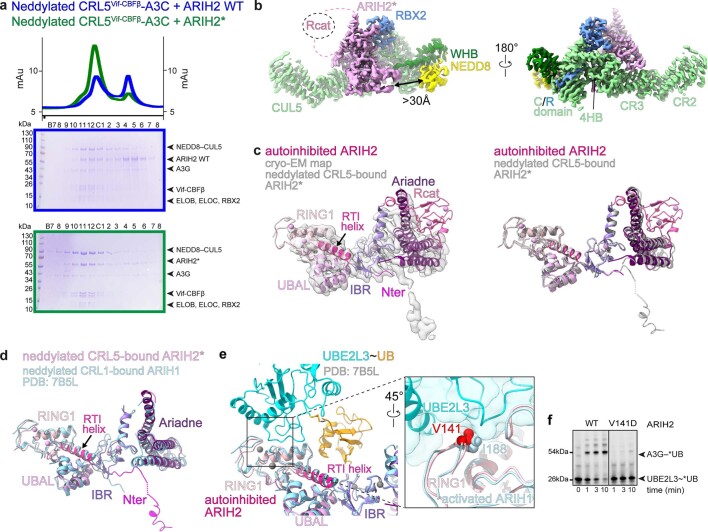
Extended Data Fig. 2. Interactions of ARIH2 and ARIH2*.
a, Chromatograms (top) and Coomassie-stained SDS-PAGE gels of fractions (bottom) from size exclusion chromatography of neddylated CRL5Vif-CBFβ–A3C mixed with WT ARIH2 (blue) or ARIH2* (green, Source Data Extended Data Fig. 2). The experiment was performed twice. b, Interactions between ARIH2* (violet) and neddylated CUL5-RBX2 seen in map from ARIH2*-neddylated CRL5Vif-CBFβ-A3C complex generated using DeepEMhancer40. Connections to the ARIH2* Rcat domain, not visible in the map, are indicated as dotted lines. Black arrow indicates the >30 Å distance between the ARIH2* UBAL domain and NEDD8. c, Left, crystal structure of autoinhibited ARIH2, with domains colored as in Fig. 1a, fit into the cryo-EM map (sharpened by DeepEMhancer40) of ARIH2* from complex with neddylated CRL5Vif-CBFβ-A3C. Right, crystal structure of autoinhibited ARIH2 with domains colored as in Fig. 1a superimposed with structure of ARIH2* (in gray) from complex with neddylated CRL5Vif-CBFβ-A3C. The Rcat domain of ARIH2* is not clearly defined in the density and thus was not modeled. To generate the model for ARIH2*, the coordinates for the UBAL, RING1, RTI-helix, and UBAL domain from the ARIH2 crystal structure were wholesale docked into the cryo EM density, which is relatively lower resolution over this region of the complex. The ARIH2* IBR and Ariadne domains were rebuilt based on the high-quality density for these regions. d, Structure of ARIH2* from complex with neddylated CRL5Vif-CBFβ-A3C, with domains colored as in Fig. 1a. Structure of corresponding region of ARIH1 (light blue) from a complex with a neddylated CRL123 is shown superimposed. The latter complex represents UB transfer from UBE2L3 to neddylated CRL1-bound ARIH1, but for simplification, the Rcat domain of ARIH1 is not shown. e, Structure of E2~UB-binding platform of autoinhibited ARIH2 is shown with domains colored as in Fig. 1a. The corresponding region of the structure representing UB transfer from UBE2L3 to neddylated CRL1-bound ARIH123 was superimposed and is shown in light blue with its bound UBE2L3~UB (cyan and orange). Close-up (rotated 45° in x) shows interface between UBE2L3 and ARIH1 RING1, highlighting the central I188, and corresponding ARIH2* RING1 V141. f, Fluorescent scan of gel showing neddylated CRL5Vif-CBFβ and ARIH2-dependent *UB transfer from UBE2L3 through E3-E3 cascade to A3G substrate, comparing WT ARIH2 and V141D mutant. (Source Data Extended Data Fig. 2). The data are representative of N = 2 independent experiments.