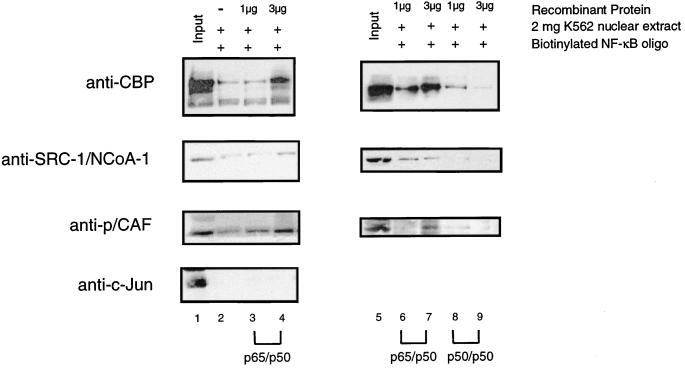
FIG. 7.
CBP/p300, SRC-1/NCoA-1, and p/CAF are recruited to a DNA-bound p65-p50 heterodimer. (Left panel) Coactivators are recruited to a DNA-bound p65-p50 heterodimer. A biotinylated oligonucleotide containing two NF-κB binding sites was bound to streptavidin-paramagnetic beads and loaded with 0 μg (lane 2), 1 μg (lane 3), or 3 μg of His-p50–His-p65 (lane 4). After a wash, the DNA-bound heterodimers were incubated with 2 mg of K562 nuclear extracts and then washed, and bound proteins were identified by Western blotting as described in Materials and Methods. Lane 1 represents 200 μg, or 1/10 of the total K562 input. The antibodies used for Western blotting are indicated on the left. (Right panel) The p50 homodimer inhibits recruitment of CBP/p300, SRC-1/NCoA-1, and p/CAF. A biotinylated oligonucleotide containing two NF-κB binding sites was bound to streptavidin-paramagnetic beads and loaded with 1 or 3 μg of either p50-p65 (lanes 6 and 7) or the p50–p50 homodimer (lanes 8 and 9). After a wash, the DNA bound dimers were incubated with 2 mg of K562 nuclear extracts and then washed, and bound proteins were identified by Western blot analysis, as described in Materials and Methods. Lane 5 (Input) represents 200 μg, or 1/10, of the total K562 input.