Abstract
This study aims to examine the impact of periodontal disease in obesity on COVID-19 infection and associated outcomes. This retrospective longitudinal study included 58,897 UK Biobank participants tested for COVID-19 between March 2020 and February 2021. Self-reported oral health indicators (bleeding gums, painful gums, and loose teeth) were used as surrogates for periodontal disease. Body fat levels were quantified by body mass index (BMI) and categorized as normal weight (18.5 to 24.9 kg/m2), overweight (25 to 29.9 kg/m2), and obese (≥30 kg/m2). Multivariable logistic regression and Cox proportional hazard models were used to quantify risk of COVID-19 infection, hospital admission, and mortality, adjusted for participants’ demographics and covariates. Of 58,897 participants, 14,466 (24.6%) tested positive for COVID-19 infection. COVID-19 infection was higher for participants who were overweight (odds ratio, 1.18; 95% CI, 1.12 to 1.24) and obese (odds ratio, 1.33; 95% CI, 1.26 to 1.41) as compared with those of normal weight, but infection was not affected by periodontal disease. The hospital admission rate was 57% higher (hazard ratio, 1.57; 95% CI, 1.25 to 1.97) in the obese group with periodontal disease than without periodontal disease, and admission rates increased with BMI category (normal weight, 4.4%; overweight, 6.8%; obese, 10.1%). Mortality rates also increased with BMI category (normal weight, 1.9%; overweight, 3.17%; obese, 4.5%). In addition, for participants with obesity, the mortality rate was much higher (hazard ratio, 3.11; 95% CI, 1.91 to 5.06) in participants with periodontal disease than those without. Obesity is associated with higher hospitalization and mortality rates, and periodontal disease may exacerbate this impact. The results could inform health providers, policy makers, and the general public of the importance to maintain good oral health through seamless provision of dental services and public oral health prevention initiatives.
Keywords: oral health, periodontal diseases, mortality, risk factors, body mass index, hospitalization
Introduction
Identification of the risk factors for severe illness or death from SARS-CoV-2 (COVID-19) is a critical step in management of the pandemic so that people at the highest risk of poor outcomes can receive targeted prevention (Lipsitch et al. 2020). Studies have shown that older age, male sex, deprivation, and comorbidities including obesity and diabetes are all risk factors for poorer COVID-19 outcomes (Chadeau-Hyam et al. 2020; Williamson et al. 2020).
The global prevalence of obesity is growing, with >1 in 4 adults in the United Kingdom living with the condition (NCD Risk Factor Collaboration 2016; NHS Digital 2020). A Public Health England report has shown that people with obesity have a greater risk of admission to the intensive care unit and mortality if they are infected with COVID-19. Oral health was not considered a covariate, and the association between COVID-19 outcomes and obesity persisted (Blackshaw et al. 2020). Furthermore, findings suggest that the association of BMI and subsequent risk of hospitalization and mortality due to COVID-19 is linear, independent of comorbidities such as diabetes (Gao et al. 2021).
There is evidence for an association between obesity and periodontal disease, although the biological basis for the association remains unclear (Chaffee and Weston 2010; Kang et al. 2019). Evidence regarding the association between oral health and COVID-19 infection is minimal, though periodontal disease shares risk factors with adverse COVID-19 outcomes, including diabetes, older age, and obesity (Sampson et al. 2020). As yet, no study has investigated the association of periodontal disease in obesity in relation to COVID-19 infection and related outcomes.
Since March 2020, the UK Biobank has provided a live feed of COVID-19 test results and linked hospital admission and mortality data, enabling extensive research on the potential risk factors and associated outcomes for COVID-19 infection and adverse outcomes (Larvin et al. 2020; Peters et al. 2021). Given the updated availability of COVID-19 test results from the UK Biobank cohort, investigation of the additive effect of periodontal disease and obesity is timely to understand the risk factors for COVID-19 infection and outcomes and to potentially reveal more about the high-risk population.
This study aims to examine the impact of periodontal disease in obesity on COVID-19 infection and associated outcomes.
Materials and Methods
Data Set
The UK Biobank cohort study contains data on >500,000 participants aged between 40 and 69 y at study recruitment (2006 to 2010), with baseline information collected during assessment center visits and self-reported responses from online questionnaires. The UK Biobank resource is linked to participant health outcomes data, incorporating ICD-10 diagnosis codes (International Statistical Classification of Diseases, Tenth Revision) and hospital admissions data from NHS Digital, in addition to death records from the death registry. Results from COVID-19 testing of UK Biobank participants have been provided by Public Health England since March 16, 2020, and represent the change from inpatient-restricted to wider community testing as national testing capacity increased. These data can be additionally accessed by approved researchers via the UK Biobank data portal according to UK Biobank guidelines (application reference 54633). Data from linked health outcomes and death records were available until November and December 2020, respectively. COVID-19 test results were available until February 2021. All data were fully anonymized by UK Biobank prior to data extraction. Participants can withdraw from the UK Biobank cohort study at any time (UK Biobank 2016).
Study Population
This longitudinal cohort study included all UK Biobank participants with a linked COVID-19 test result and oral health status available, as well as body mass index (BMI) ≥18.5 kg/m2 (n = 58,897). The applied study inclusion and exclusion criteria are demonstrated in a flow chart (Appendix Fig.).
The final data set utilized for this study encompassed information on participant demographics, comorbidities, and health outcomes data including hospital admissions and death records.
Study Outcomes
The primary outcomes for this cohort study were COVID-19 infection and associated adverse outcomes: subsequent hospital admission and mortality at 1 mo following a positive COVID-19 test result. In cases where participants had multiple reported test results available, the first positive test result was used as the index event; if a participant presented only negative COVID-19 test results, the first test was used as the index event.
Study Exposures
Periodontal Disease
Self-reported oral health indicators were used as surrogates for periodontal disease (Abbood et al. 2016). Participants were asked if they had any of a number of dental problems. Bleeding gums and painful gums were used as surrogates for mild to moderate periodontal disease, while self-reported loose teeth were indicative of severe periodontal disease (Larvin et al. 2021). In cases of multiple responses, the most severe indicator was used as the primary surrogate for periodontal disease. Participants who did not report any of the oral health indicators were deemed to have no history of periodontal disease and were periodontally healthy at baseline.
Body Mass Index
Obesity status was quantified by BMI. A BMI of 18.5 to 24.9 kg/m2 was classified as normal weight, 25 to 29.9 kg/m2 as overweight, and ≥30 kg/m2 as obese (World Health Organization 2020).
Covariates
Baseline information—including age, sex, ethnicity, household income, BMI, blood pressure, C-reactive protein level, and self-reported oral health indicators—was collected during visits to UK Biobank assessment centers. History of smoking was derived from self-report and dichotomized as current or ex-smoker. Medical history was collected during assessment center visits or extracted from validated ICD-10 codes in health outcomes data. Comorbidities included cancer, diabetes, respiratory disease, inflammatory disease, and cardiovascular disease conditions (hypertension, stroke, myocardial infarction, peripheral artery disease heart failure, and atrial fibrillation). The CALIBER resource (Cardiovascular Research Using Linked Bespoke Studies and Electronic Health Records) was used to identify conditions through ICD-10 codes and the associated self-reported condition (Denaxas et al. 2012; Appendix Table 1). History of hypertension was determined by self-report, ICD-10 diagnosis code identification in health outcomes data, or a blood pressure reading that exceeded 140/90 mm Hg.
Statistical Analysis
Baseline characteristics were presented by frequency (percentage) and mean (standard deviation) for categorical and continuous variables, respectively. Logistic regression was used to quantify the risk of COVID-19 infection stratified by periodontal disease and BMI category, adjusted for demographics and covariates. Subsequent hospital admission and mortality within 1 month of COVID-19 infection were quantified with Cox proportional hazard models. Estimates were adjusted for covariates including age, sex, household income, C-reactive protein level, blood pressure, history of smoking, and comorbidities. To assess the additive effect of periodontal disease and obesity on COVID-19 outcomes, each model was performed by stratification of periodontal disease status and BMI category, respectively. Odds ratio (OR) or hazard ratio (HR) was reported for each oral health indicator and a combined effect for periodontal disease of the stratified categories.
Overall rates of COVID-19 infection, hospital admission, and mortality were plotted against periodontal status and BMI category. With regard to missing data, multiple imputations were conducted, and coefficients were combined according to Rubin’s (2004) rule. Sensitivity analyses were also conducted with only complete cases to account for the impact of missing data and stratification by additional obesity categories (category 1, BMI = 30 to 34.9 kg/m2; category 2, BMI >35 kg/m2). The statistical significance level was set to 0.05. Data processing and analyses were performed with R version 4.0.0 (R Core Team 2017). This report conforms to STROBE guidelines for cohort studies.
Results
A total of 58,897 participants were included in the final study cohort, 14,466 (24.6%) of which were confirmed COVID-19 cases (Appendix Fig.). The average age of all participants was 56.83 y (SD, 8.33); 31,489 (53.5%) were female; and 54,849 (93.4%) were White (Table 1). There were 6,124 (10.3%) participants with bleeding gums, 1,511 (2.5%) with loose teeth, 1,397 (2.4%) with painful gums, and the remaining 49,565 (84.1%) did not report periodontal disease. Of participants with self-reported periodontal disease, 2,401 (26.5%) were of normal weight status, 3,705 (41.0%) overweight, and 2,926 (32.3%) obese. Baseline characteristics of study participants across oral health indicators are presented in Appendix Table 2.
Table 1.
Baseline Characteristics of Study Participants Stratified by BMI Category and PD Status.
| Normal Weight (18.5 to 24.9 kg/m2) | Overweight (25 to 29.9 kg/m2) | Obese (≥30 kg/m2) | |||||
|---|---|---|---|---|---|---|---|
| Overall | Healthy | Any PD | Healthy | Any PD | Healthy | Any PD | |
| Participants, n | 58,897 | 14,701 | 2,401 | 21,365 | 3,705 | 13,799 | 2,926 |
| Age, y, mean (SD) | 56.83 (8.33) | 56.13 (8.54) | 54.67 (8.17) | 57.59 (8.28) | 55.86 (8.24) | 57.39 (8.06) | 55.19 (8.12) |
| Sex: female | 31,489 (53.5) | 9,489 (64.5) | 1,692 (70.5) | 9,726 (45.5) | 1,962 (53.0) | 7,009 (50.8) | 1,611 (55.1) |
| Ethnicity: White | 54,849 (93.4) | 13,850 (94.4) | 2,176 (90.8) | 20,072 (94.2) | 3,312 (89.6) | 12,852 (93.4) | 2,587 (88.7) |
| Household income, £ | |||||||
| <18,000 | 12,520 (25.0) | 2,607 (20.7) | 475 (23.1) | 4,288 (23.5) | 811 (25.6) | 3,523 (30.5) | 816 (33.1) |
| 18,000 to 30,999 | 12,662 (25.3) | 3,061 (24.3) | 493 (23.9) | 4,662 (25.5) | 834 (26.3) | 2,964 (25.6) | 648 (26.3) |
| 31,000 to 51,999 | 12,771 (25.5) | 3,189 (25.3) | 564 (27.4) | 4,766 (26.1) | 823 (26.0) | 2,851 (24.7) | 578 (23.5) |
| 52,000 to 100,000 | 9,454 (18.9) | 2,748 (21.8) | 422 (20.5) | 3,532 (19.3) | 556 (17.6) | 1,845 (16.0) | 351 (14.2) |
| >100,000 | 2,683 (5.4) | 975 (7.8) | 106 (5.1) | 1,009 (5.5) | 142 (4.5) | 380 (3.3) | 71 (2.9) |
| BMI, mean (SD) | 26.84 (4.38) | 22.90 (1.53) | 22.91 (1.53) | 27.35 (1.41) | 27.42 (1.40) | 34.09 (3.98) | 34.48 (4.32) |
| CRP, mm Hg, mean (SD) | 2.78 (4.47) | 1.76 (3.77) | 1.84 (4.20) | 2.51 (4.08) | 2.57 (3.94) | 4.19 (5.22) | 4.35 (5.37) |
| Blood pressure, mean (SD) | |||||||
| Systolic | 139.69 (19.68) | 134.78 (19.93) | 132.42 (19.60) | 141.18 (19.30) | 139.32 (19.36) | 143.72 (18.82) | 141.13 (18.90) |
| Diastolic | 82.19 (10.69) | 78.52 (10.38) | 78.06 (10.34) | 82.56 (10.30) | 82.38 (10.19) | 85.53 (10.39) | 85.41 (10.47) |
| Ever smoked | 36,086 (87.7) | 8,698 (83.7) | 1,495 (86.4) | 13,123 (87.9) | 2,400 (91.0) | 8,483 (90.0) | 1,887 (92.5) |
| Medical history | |||||||
| Cancer | 6,294 (10.7) | 1,587 (10.8) | 268 (11.2) | 2,267 (10.6) | 383 (10.3) | 1,473 (10.7) | 316 (10.8) |
| CVD | 12,208 (20.7) | 3,043 (20.7) | 506 (21.1) | 4,459 (20.9) | 746 (20.1) | 2,825 (20.5) | 629 (21.5) |
| Diabetes | 3,530 (6.0) | 906 (6.2) | 136 (5.7) | 1,258 (5.9) | 223 (6.0) | 836 (6.1) | 171 (5.8) |
| Hypertension | 18,439 (31.3) | 4,527 (30.8) | 746 (31.1) | 6,824 (31.9) | 1,165 (31.4) | 4,459 (20.9) | 746 (20.1) |
| Inflammatory disease | 2,342 (4.0) | 622 (4.2) | 92 (3.8) | 806 (3.8) | 170 (4.6) | 537 (3.9) | 115 (3.9) |
| Respiratory disease | 11,472 (19.5) | 2,786 (19.0) | 473 (19.7) | 4,218 (19.7) | 732 (19.8) | 2,694 (19.5) | 569 (19.4) |
Values are presented as No. (%) or mean (SD) as indicated. Means and percentages are calculated for variables excluding missing data. There were missing data in the following variables: ethnicity (0.3%), CRP (6.0%), household income (15.0%), BMI (3.0%), systolic and diastolic blood pressure readings (3.4%), and history of smoking (30.1%).
BMI, body mass index; CRP, C-reactive protein; CVD, cardiovascular disease; PD, periodontal disease.
COVID-19 Infection
The rates of COVID-19 infection for participants without periodontal disease were 22.0%, 23.9%, and 26.5% for normal weight, overweight, and obese status, respectively (Table 2, Fig. 1). The infection rates for participants with periodontal disease in these categories were 25.9%, 27.1%, and 28.7%.
Table 2.
Rates of COVID-19 Infection and Hospital Admission and Mortality Within 1 mo of a Positive COVID-19 Test Result in Study Participants Stratified by BMI Category and Oral Health Indicator.
| Normal Weight (18.5 to 24.9 kg/m2) | Overweight (25 to 29.9 kg/m2) | Obese (≥30 kg/m2) | |||||
| Overall | Healthy | Any PD | Healthy | Any PD | Healthy | Any PD | |
| Participants, n | 58,897 | 14,701 | 2,401 | 21,365 | 3,705 | 13,799 | 2,926 |
| Positive COVID-19 test result | 14,466 (24.6) | 3,234 (22.0) | 622 (25.9) | 5,111 (23.9) | 1,005 (27.1) | 3,654 (26.5) | 840 (28.7) |
| Within 1 mo of positive test result | |||||||
| Hospital admission | 1,041 (7.2) | 145 (4.5) | 24 (3.9) | 343 (6.7) | 73 (7.3) | 372 (10.2) | 84 (10.0) |
| Mortality | 469 (3.2) | 68 (2.1) | 5 (0.8) | 169 (3.3) | 25 (2.5) | 172 (4.7) | 30 (3.6) |
Values are presented as No. (%). Rates of hospital admission and mortality are based on number of participants with a positive COVID-19 test result.
BMI, body mass index; PD, periodontal disease.
Figure 1.
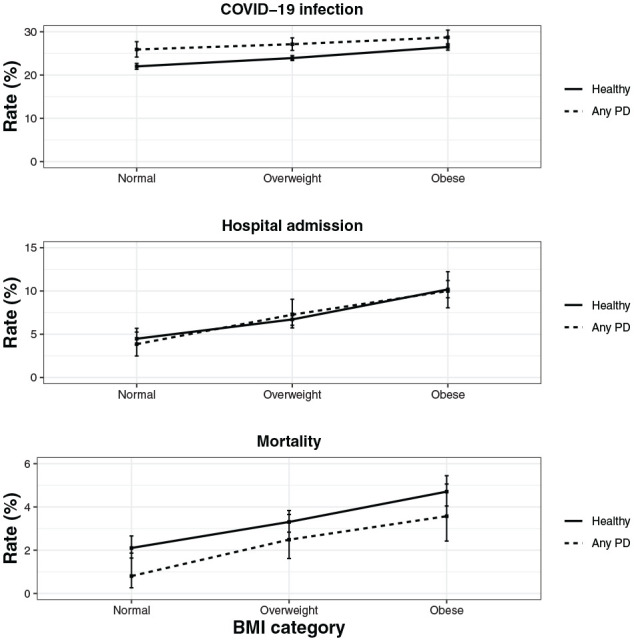
The rate of COVID-19 infection, hospital admission, and mortality by oral health status and BMI category. Error bars indicate 95% CI. BMI, body mass index; PD, periodontal disease.
Healthy Controls
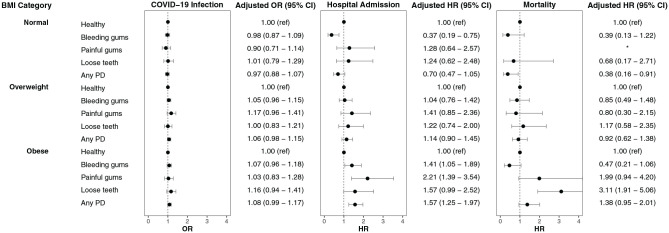
The risk of infection in individuals without periodontal disease was higher in those who were overweight (OR, 1.18; 95% CI, 1.12 to 1.24) and obese (OR, 1.33; 95% CI, 1.26 to 1.41) than in those of normal weight (Fig. 2).
Figure 2.
Adjusted odds ratios (ORs) and hazard ratios (HRs) show risk of COVID-19 infection and hospital admission and mortality within 1 mo for participants with COVID-19 across body mass index categories and stratified by oral health indicators as compared with healthy controls. PD, periodontal disease. *Insufficient data available.
People with Periodontal Disease
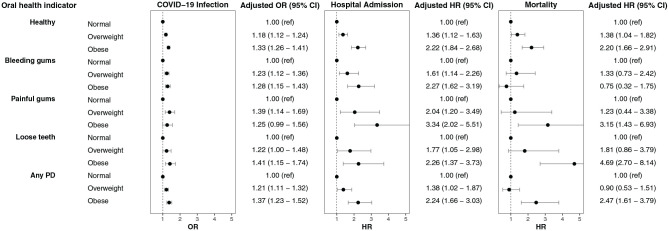
After adjustment for covariates, the risk for infection in individuals with periodontal disease was not different compared to participants without periodontal disease across the 3 BMI categories: normal weight (OR, 0.97; 95% CI, 0.88 to 1.07), overweight (OR, 1.06; 95% CI, 0.98 to 1.15), and obese (OR, 1.08; 95% CI, 0.99 to 1.17) (Fig. 3). The risk for infection in individuals with periodontal disease was higher in participants who were overweight (OR, 1.21; 95% CI, 1.11 to 1.32) and obese (OR, 1.37; 95% CI, 1.23 to 1.52) than in participants of normal weight (Fig. 2).
Figure 3.
Adjusted odds ratios (ORs) and hazard ratios (HRs) show risk of COVID-19 infection and hospital admission and mortality within 1 mo for participants with COVID-19 across oral health indicators and stratified by BMI category as compared with healthy controls. BMI, body mass index; PD, periodontal disease.
Hospital Admission
The rates of hospital admission within 1 mo of a COVID-19–positive test for participants without periodontal disease were 4.5%, 6.7%, and 10.2% for normal weight, overweight, and obese status, respectively (Table 2, Fig. 1). The admission rates for participants with periodontal disease were 3.9%, 7.3%, and 10.0%.
Healthy Controls
The risk for hospital admission in people without periodontal disease was 36% higher in those who were overweight (HR, 1.36; 95% CI, 1.12 to 1.63) and 122% higher in those who were obese (HR, 2.22; 95% CI, 1.84 to 2.68) compared to those of normal weight (Fig. 2).
People with Periodontal Disease
After adjustment for covariates, the risk of hospital admission due to periodontal disease was no different for participants of normal weight (HR, 0.70; 95% CI, 0.47 to 1.05) or overweight status (HR, 1.14; 95% CI, 0.90 to 1.45) but was higher in those who were obese (HR, 1.57; 95% CI, 1.25 to 1.97; Appendix Table 3, Fig. 3). The risk of hospital admission for people with periodontal disease was 38% higher in participants who were overweight (HR, 1.38; 95% CI, 1.02 to 1.87) and 124% higher in those who were obese (HR, 2.24; 95% CI, 1.66 to 3.03) compared to those of normal weight (Fig. 2).
Mortality
The median survival time between a COVID-19–positive test result and mortality was 10 d (interquartile range, 5 to 21 d). The 1-mo mortality rates after a COVID-19–positive test result for participants without periodontal disease were 2.1%, 3.3%, and 4.7% for those of normal weight, overweight, and obese status, respectively (Table 2, Fig. 1). The mortality rates for participants with periodontal disease were 0.8%, 2.5%, and 3.6%.
Healthy Controls
The 1-mo mortality risk for people without periodontal disease was 38% higher in the overweight group (HR, 1.38; 95% CI, 1.04 to 1.82) and 120% higher in the obese group (HR, 2.20; 95% CI, 1.66 to 2.91) than in the normal weight group (Fig. 2).
People with Periodontal Disease
After adjustment for covariates, the risk for mortality due to periodontal disease was no different across BMI categories (Fig. 3). It is worth noting that participants with loose teeth exhibited much higher mortality in the obese category (HR, 3.11; 95% CI, 1.91 to 5.06; Appendix Table 4; Fig. 2). The mortality rate for people with periodontal disease was no different in those who were overweight (HR, 0.90; 95% CI, 0.53 to 1.51), but it was 147% higher in participants who were obese (HR, 2.47; 95% CI, 1.61 to 3.79) compared to those of normal weight.
Sensitivity analysis showed similar effects to risk estimates for COVID-19 infection and associated outcomes in complete cases only (Appendix Tables 5 and 6). Risk estimates for COVID-19 infection and associated outcomes based on additional obesity categories reflected similar results to those observed in Figures 2 and 3 (Appendix Tables 7 and 8).
Discussion
This study is the first of its kind to assess the additive impact of obesity and periodontal disease on COVID-19 infection and associated adverse outcomes. Individuals who were overweight or obese were more likely to be infected with COVID-19 and more likely to be hospitalized or die within 1 mo of infection than people of normal weight. In addition, people with obesity and periodontal disease were more likely to be hospitalized or die with the infection than those who were obese and did not report periodontal disease. While obesity has a larger impact on COVID-19 infection and associated outcomes, periodontal disease may augment the risk of hospital admission and mortality in people classified as overweight or obese.
Our findings are consistent with existing studies that suggest that obesity may increase infection and cause adverse outcomes (Chandarana et al. 2021; Freuer et al. 2021). A modest additive effect of periodontal disease in obese people on their risks of hospital admission and mortality following COVID-19 infection was observed. A case-control study on a smaller sample (n = 568) demonstrated a higher risk of mortality and hospitalization in people with periodontitis, although the model did not adjust for BMI and the nature of the study design prevents the ability to infer causation (Marouf et al. 2021). Interestingly, our earlier retrospective study utilizing a smaller UK Biobank cohort sample showed a higher risk of mortality among people with periodontal disease. We did not previously observe higher risk in hospital admission; this could have been due to the smaller sample size at the time of writing (Larvin et al. 2021). Differences in magnitude of the associations in the present study based on an updated UK Biobank dataset suggests possible differences in the effect of risk factors between the first and second waves of the COVID-19 pandemic.
Previous research has demonstrated the association of periodontal disease and obesity (Jepsen et al. 2020). The modest augmented risk of poorer outcomes associated with COVID-19 infection observed in people with higher BMI and periodontal disease could be due to chronic inflammation from the combined conditions causing more severe COVID-19 progression. Augmented levels of C-reactive protein, a marker of chronic inflammation, have been observed in patients hospitalized with COVID-19 (McNeill et al. 2021). This marker is also increased in people with severe periodontal disease (Linden et al. 2008), with amplified levels in obese men with periodontitis (Meisel et al. 2019). A mendelian randomization study has already implicated a causal link between obesity and poorer COVID-19 outcomes (Freuer et al. 2021). The findings of the present study suggest that inflammation caused by severe periodontal disease may exacerbate the causal effect of higher BMI and increased likelihood of COVID-19 hospitalization.
Additional studies are required to confirm the potentially additive effect of periodontal disease in obesity on risk of mortality. While rates for mortality appeared lower in people with periodontal disease, the direction of risk was reversed following adjustment for covariates in the obese group with periodontal disease but not for people in the normal and overweight groups. This may be due to the lower average age observed in people with periodontal disease overall, and it suggests that periodontal disease in the obese group has a greater impact on mortality than in the normal and overweight groups. Lower mortality estimates may have also been caused by combining the oral health indicators, as people with bleeding gums seemed to have lower rates of mortality than those with loose teeth. Mehta and Preston (2016) speculate that as obesity is usually developed in midlife and not a fixed trait, the additive effect observed for obesity with other risk factors is caused by subordinate interactions. If left untreated, periodontal disease also progresses over time and has a higher prevalence in older age (López et al. 2017). As the UK Biobank comprises predominantly middle-aged people, it is possible that the additive effect of periodontal disease and obesity observed in the present study is due to sociodemographic or other behavioral factors as opposed to physiologic interaction (Mehta and Preston 2016). Given that mortality is the most severe outcome of COVID-19, it is likely that the trajectory from COVID-19 infection to mortality is more multifactorial than other outcomes and could explain the difference in the effects observed on COVID-19 infection and hospitalization.
Whereas BMI is frequently accounted for in epidemiologic studies, our findings suggest that future research should incorporate oral health status as a covariate in risk models for COVID-19 infection and associated outcomes. Additionally, future research should account for the date of COVID-19 infection and associated outcomes or the effect on outcomes of specific COVID-19 strains. This would improve understanding of risk factors for the prevalent circulating strains and reveal those most vulnerable to the consequences of the viral mutations between pandemic waves. The general public should be made aware of the additive impact of poor oral health and high BMI for severe COVID-19 progression and associated adverse outcomes. Additionally, good oral hygiene and self-maintenance should be encouraged as a public health initiative in preventing poorer COVID-19 outcomes, particularly while dental services are limited during the pandemic.
The strengths elicited from this study include the utilization of UK Biobank data. The UK Biobank provides a unique cohort to examine the effect of a number of exposures and covariates; linked health outcomes data and death records make it an invaluable tool for epidemiologic research. As a result of linked COVID-19 test results, we were able to compare infection rates using negative and positive test results, which is a rarity in the compiling COVID-19 research. Using the updated, larger COVID-19 test results data set, we were able to make comparisons, draw on conclusions from our previous study, and combine the findings with other studies linking COVID-19 and obesity.
The findings of this study were limited by the use of self-reported oral health indicators, as these may be subject to reduced sensitivity and specificity (Montero et al. 2020). Accuracy of self-reported periodontal disease may also be dependent on population demographics (Blicher et al. 2005). Conversely, it has been suggested that self-reported bleeding gums, painful gums, and loose teeth can accurately identify periodontal disease cases; however, more evidence is required to confirm this (Abbood et al. 2016). A clinical classification of periodontal disease via linked medical and dental records would replace the use of self-reported indicators and negate the associated biases. Random sampling for people in the present study was not applicable due to the nature of the data linkage. Overall, 15% of the sample population had self-reported periodontal disease; 7% had bleeding gums; and 2% and 3% reported painful gums and loose teeth. The Adult Dental Health Survey 2009 found that 54% adults report bleeding gums, indicative of milder periodontal disease, while 45% have chronic periodontitis (White et al. 2011). As prevalence of periodontal disease is lower in the study sample, it is possible that the findings are subject to some selection bias and should be interpreted cautiously. Furthermore, given the limitations of traditional epidemiologic multivariate modeling and potential confounding bias, causal inferences between 1) obesity, periodontal disease, and COVID-19 infection and 2) subsequent adverse outcomes should be interpreted with caution. Alternate methods of causal modeling, such as marginal structural models, could be used to reduce confounding and assess mediation effects (Robins et al. 2000); however, such techniques could still be limited by data collection and selection bias. Further analyses are also required to investigate the underlying mechanisms that lead to the potential interactions among obesity, periodontal disease, and COVID-19.
Conclusions
Obesity had a more significant impact on infection and adverse COVID-19 outcomes than periodontal disease. Our study revealed that periodontal disease may exacerbate the effect of obesity on hospitalization and mortality following COVID-19 infection, although further investigation is required. Increased public and professional awareness of this potential risk could be informative for future pandemic policy preparations regarding comorbidities and their impact to health outcomes, including prioritization of oral health maintenance through seamless provision of dental services and public oral health prevention initiatives.
Author Contributions
H. Larvin, contributed to conception, data analysis, and interpretation, drafted and critically revised the manuscript; S. Wilmott, contributed to data interpretation, drafted and critically revised the manuscript; J. Kang, V.R. Aggarwal, contributed to data interpretation, critically revised the manuscript; S. Pavitt, contributed to conception and data interpretation, critically revised the manuscript; J. Wu, contributed to conception, design, data acquisition, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345211029638 for Additive Effect of Periodontal Disease and Obesity on COVID-19 Outcomes by H. Larvin, S. Wilmott, J. Kang, V.R. Aggarwal, S. Pavitt and J. Wu in Journal of Dental Research
Acknowledgments
This research has been conducted with data from UK Biobank, a major biomedical database under protocol reference 54633.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: H. Larvin was supported by the Hopper Scholarship at the University of Leeds. This work was supported by the National Institute for Health Research infrastructure at Leeds. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
ORCID iDs: H. Larvin  https://orcid.org/0000-0001-7263-4182
https://orcid.org/0000-0001-7263-4182
References
- Abbood HM, Hinz J, Cherukara G, Macfarlane TV. 2016. Validity of self-reported periodontal disease: a systematic review and meta-analysis. J Periodontol. 87(12):1474–1483. [DOI] [PubMed] [Google Scholar]
- Blackshaw J, Feeley A, Mabbs L, Niblett P, Atherton E, Elsom R, Hung E, Tedstone A.2020. Excess weight and COVID-19: insights from new evidence. London (UK): PHE Publications. [Google Scholar]
- Blicher B, Joshipura K, Eke P.2005. Validation of self-reported periodontal disease: a systematic review. J Dent Res. 84(10):881–890. [DOI] [PubMed] [Google Scholar]
- Chadeau-Hyam M, Bodinier B, Elliott J, Whitaker MD, Tzoulaki I, Vermeulen R, Kelly-Irving M, Delpierre C, Elliott P.2020. Risk factors for positive and negative COVID-19 tests: a cautious and in-depth analysis of UK Biobank data. Int J Epidemiol. 49(5):1454–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee BW, Weston SJ. 2010. Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol. 81(12):1708–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarana H, Dane B, Mikheev A, Taffel MT, Feng Y, Rusinek H.2021. Visceral adipose tissue in patients with COVID-19: risk stratification for severity. Abdom Radiol (NY). 46(2):818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaxas SC, George J, Herrett E, Shah AD, Kalra D, Hingorani AD, Kivimaki M, Timmis AD, Smeeth L, Hemingway H.2012. Data resource profile: cardiovascular disease research using linked bespoke studies and electronic health records (CALIBER). Int J Epidemiol. 41(6):1625–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freuer D, Linseisen J, Meisinger C.2021. Impact of body composition on COVID-19 susceptibility and severity: a two-sample multivariable mendelian randomization study. Metabolism. 118:154732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O’Rahilly S, Aveyard P, Jebb SA. 2021. Associations between body-mass index and COVID-19 severity in 6.7 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 9(6):350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen S, Suvan J, Deschner J.2020. The association of periodontal diseases with metabolic syndrome and obesity. Periodontol 2000. 83(1):125–153. [DOI] [PubMed] [Google Scholar]
- Kang J, Smith S, Pavitt S, Wu J.2019. Association between central obesity and tooth loss in the non-obese people: results from the continuous National Health and Nutrition Examination Survey (NHANES) 1999–2012. J Clin Periodontol. 46(4):430–437. [DOI] [PubMed] [Google Scholar]
- Larvin H, Kang J, Aggarwal VR, Pavitt S, Wu J.2021. Risk of incident cardiovascular disease in people with periodontal disease: a systematic review and meta-analysis. Clin Exp Dent Res. 7(1):109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larvin H, Wilmott S, Wu J, Kang J.2020. The impact of periodontal disease on hospital admission and mortality during COVID-19 pandemic. Front Med. 7:861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden GJ, McClean K, Young I, Evans A, Kee F.2008. Persistently raised C-reactive protein levels are associated with advanced periodontal disease. J Clin Periodontol. 35(9):741–747. [DOI] [PubMed] [Google Scholar]
- Lipsitch M, Swerdlow DL, Finelli L.2020. Defining the epidemiology of COVID-19—studies needed. N Engl J Med. 382(13):1194–1196. [DOI] [PubMed] [Google Scholar]
- López R, Smith PC, Göstemeyer G, Schwendicke F.2017. Ageing, dental caries and periodontal diseases. J Clin Periodontol. 44 Suppl 18:S145–S152. [DOI] [PubMed] [Google Scholar]
- Marouf N, Cai W, Said KN, Daas H, Diab H, Chinta VR, Hssain AA, Nicolau B, Sanz M, Tamimi F.2021. Association between periodontitis and severity of COVID-19 infection: a case-control study. J Clin Periodontol. 48(4):483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill JN, Lau ES, Paniagua SM, Liu EE, Wang JK, Bassett IV, Selvaggi CA, Lubitz SA, Foulkes AS, Ho JE. 2021. The role of obesity in inflammatory markers in COVID-19 patients. Obes Res Clin Pract. 15(1):96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N, Preston S.2016. Are major behavioral and sociodemographic risk factors for mortality additive or multiplicative in their effects? Soc Sci Med. 154:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel P, Eremenko M, Holtfreter B, Völzke H, Kocher T.2019. The sex paradox in the interplay between periodontitis, obesity, and serum C-reactive protein: data from a general population. J Periodontol. 90(12):1365–1373. [DOI] [PubMed] [Google Scholar]
- Montero E, La Rosa M, Montanya E, Calle-Pascual AL, Genco RJ, Sanz M, Herrera D.2020. Validation of self-reported measures of periodontitis in a Spanish population. J Periodontal Res. 55(3):400–409. [DOI] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration. 2016. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 387(10026):1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS Digital. 2020. Statistics on obesity, physical activity and diet. England (UK): NHS Digital. [Google Scholar]
- Peters SAE, MacMahon S, Woodward M. 2021. Obesity as a risk factor for COVID-19 mortality in women and men in the UK Biobank: comparisons with influenza/pneumonia and coronary heart disease. Diabetes Obes Metab. 23(1):258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2017. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. [Google Scholar]
- Robins JM, Hernán MA, Brumback B.2000. Marginal structural models and causal inference in epidemiology. Epidemiology. 11(5):550–560. [DOI] [PubMed] [Google Scholar]
- Rubin DB.2004. Multiple imputation for nonresponse in surveys. Hoboken (NJ): John Wiley & Sons. [Google Scholar]
- Sampson V, Kamona N, Sampson A.2020. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br Dent J. 228(12):971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Biobank. 2016. Protocol for a large-scale prospective epidemiological resource [accessed 2021. Jun 14]. https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/uk-biobank-a-large-scale-prospective-epidemiological-resource/
- White D, Pitts N, Steele J, Sadler K, Chadwick B.2011. Disease and related disorders—a report from the Adult Dental Health Survey 2009. England (UK): NHS Digital. [Google Scholar]
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, et al. 2020. Factors associated with COVID-19–related death using OpenSAFELY. Nature. 584(7821):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation. 2020. Obesity and overweight [accessed 2021. Jun 14]. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345211029638 for Additive Effect of Periodontal Disease and Obesity on COVID-19 Outcomes by H. Larvin, S. Wilmott, J. Kang, V.R. Aggarwal, S. Pavitt and J. Wu in Journal of Dental Research