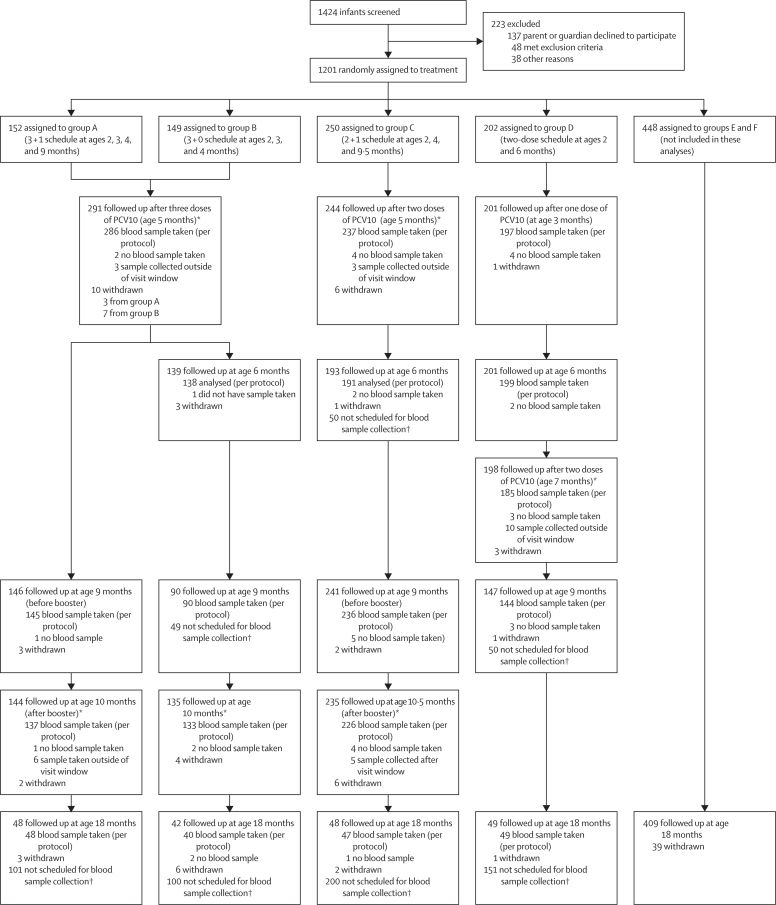
Figure 1.
Trial profile
Samples collected outside the visit window (27–43 days after vaccination) were included only in the intention-to-treat analyses. Full details of the trial have been described previously.15 *Half of participants in groups A–D contributed to the opsonophagocytic assay analyses. †Participants allocated to groups A–D from the last 300 recruited provided a blood sample at age 18 months, with the remainder providing a sample at an alternative timepoint, because a maximum of four blood samples was permitted per participant over the course of the study.