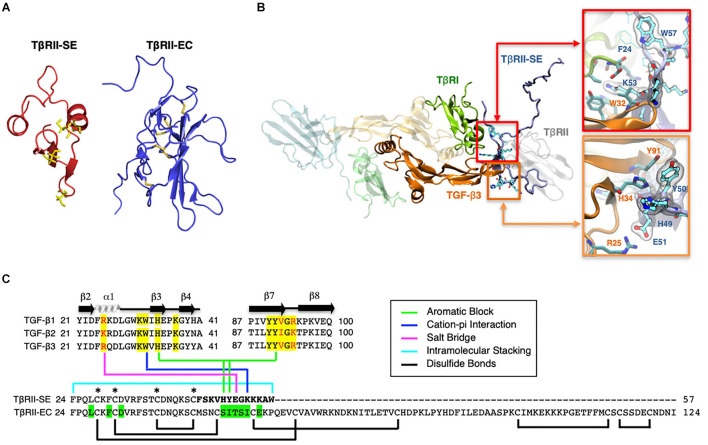
FIGURE 2.
3D modeling reveals unique characteristics of TβRII-SE in comparison to the extracellular domain of membrane-bound TβRII. (A) TβRII-SE and TβRII-EC 3D models showing cysteine residues in yellow, and lack of disulfide bonds in TβRII-SE. (B) 3D model of mature TβRII-SE superimposed over the crystallographic structure of 2PJY (TβRII/TβRI/TGF-β3) (left panel). The amino acid interactions between TβRII-SE (blue residues) and TGF-β3 (orange residues) are shown magnified in the right upper and lower panels. (C) Amino acid sequence alignment of human TGF-β family members (top panel) showing residues interacting with TβRII-EC (yellow shading) and residues interacting with TβRII-SE (color lines linking the bottom panel). Green shading indicates residues in TβRII-EC interacting with TGF-β cognate ligands. Brackets and asterisks indicate cysteine residues forming and not forming disulfide bonds in TβRII-EC and TβRII-SE, respectively.