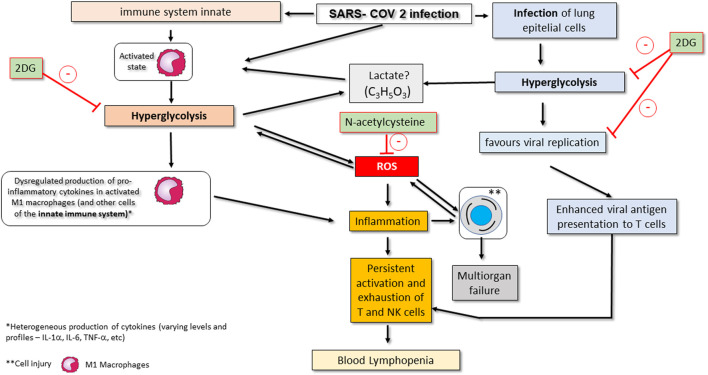
FIGURE 2.
Conceptual model of SARS-CoV-2-associated changes in the glycolytic pathway and its consequences on COVID-19 in critically ill patients. Viral infection activates the innate immune system, namely M1 macrophages. Activation of macrophages promotes metabolic changes for their effective action, including an increase in consumption of glycolysis (hyperglycolysis) and a change in the strategy for ATP synthesis (metabolic reprogramming). These changes favour the production of ROS and worsen inflammation. Hyperglycolysis may also promote an increase in serum lactate. SARS-CoV-2 infection also affects epithelial lung cells by making them increase their glucose consumption, which makes viral replication viable. Infected epithelial cells also show enhanced presentation of viral antigens to T lymphocytes (particularly CD8+ T cells), and NK cells are also affected, with both cell types becoming more chronically activated. These events promote exhaustion and subsequent apoptosis of T and NK cells, thereby contributing to peripheral lymphopenia. Drugs that may eventually be used as complementary treatment of severe COVID-19 infection by inhibition infection-associated hyperglycolysis include 2-deoxy-d-glucose (2DG), N-acetylcysteine or GLUT1 inhibitors. Administration of 2DG inhibits hyperglycolysis in innate immune system cells such as macrophages; on the other hand, the administration of N -acetylcysteine inhibits the increase in ROS production by activating glutathione (see therapeutic strategies section). The action of GLUT1 inhibitors is not shown but involves blocking glucose uptake in activated and infected immune system and epithelial cells.