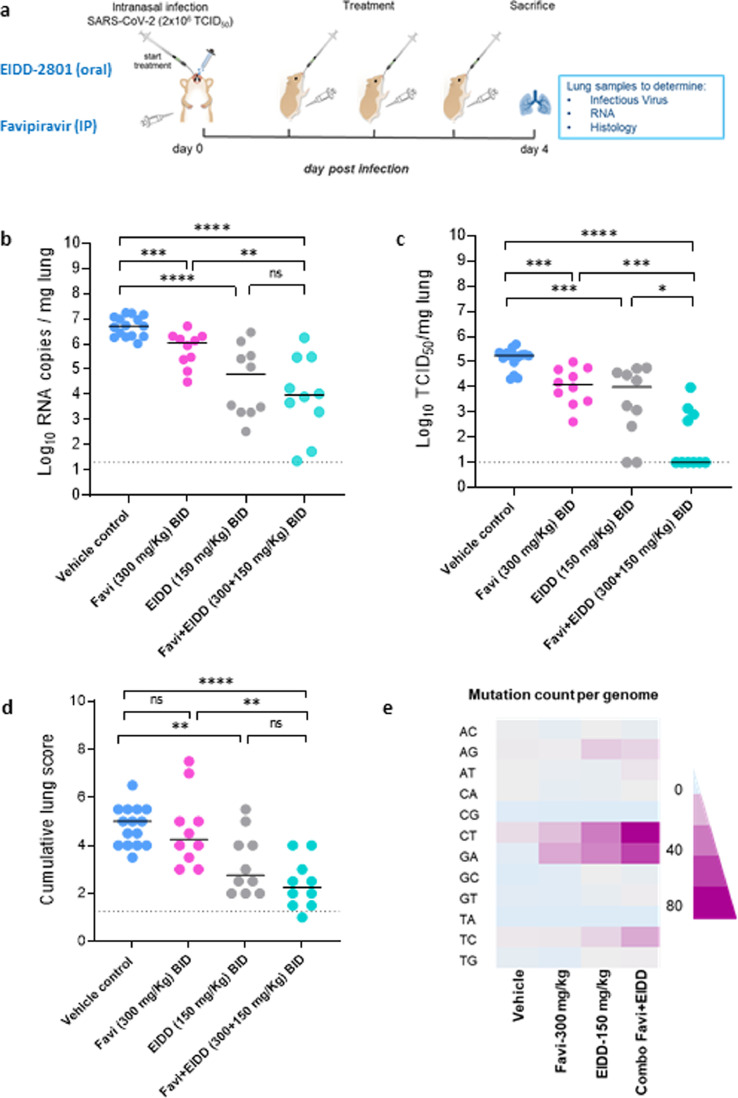
Fig. 1.
Combined efficacy of Favipiravir and Molnupiravir (EIDD-2801) against SARS-CoV-2 in a hamster infection model. (a) Set-up of the study. (b) Viral RNA levels in the lungs of control (vehicle-treated), Favipiravir-treated (300 mg/kg, BID), EIDD-2801-treated (150 mg/kg, BID) and combination-treated (Favipiravir+EIDD-2801 at 300+150 mg/kg, BID, respectively) SARS-CoV-2−infected hamsters at day 4 post-infection (pi) are expressed as log10 SARS-CoV-2 RNA copies per mg lung tissue. Individual data and median values are presented. (c) Infectious viral loads in the lungs of control (vehicle-treated), Favipiravir-treated, EIDD-2801-treated and combination-treated (Favipiravir+EIDD-2801) SARS-CoV-2−infected hamsters at day 4 pi are expressed as log10 TCID50 per mg lung tissue. Individual data and median values are presented. (d) Cumulative severity score from H&E stained slides of lungs from control (vehicle-treated), Favipiravir-treated, EIDD-2801-treated and combination-treated (Favipiravir+EIDD-2801) SARS-CoV-2−infected hamsters. Individual data and median values are presented and the dotted line represents the median score of untreated non-infected hamsters. (e) Mean mutation count (per the whole genome) in the viral RNA isolated from the lungs of control (vehicle-treated), Favipiravir-treated (300 mg/kg, BID), EIDD-2801-treated (150 mg/kg, BID) and combination-treated (Favipiravir+EIDD-2801 at 300+150 mg/kg, BID, respectively) SARS-CoV-2−infected hamsters at day 4 post-infection (pi). Data were analyzed with the Mann−Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns=non-significant. Favi=Favipiravir, EIDD=EIDD-2801. All data (panels B, C, D) are from two independent experiments with 15, 10, 10 and 10 animals for respectively the vehicle, Favipiravir 300 mg/kg, EIDD-2801 150 mg/kg and Favipiravir+EIDD-2801 condition.