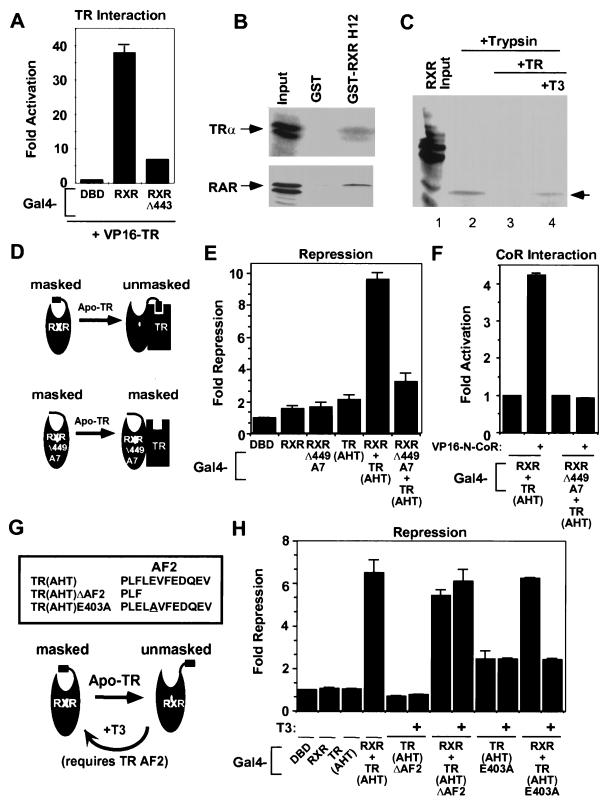
FIG. 5.
Unmasking the CoR interaction domain of RXR by “H12 docking” with a heterodimer partner. (A) RXR H12 contributes to the affinity of RXR for TR: mammalian two-hybrid assay with Gal4-RXR or Gal4-RXRΔ449 and VP16-N-CoR. (B) RXR H12 interacts specifically with apo-TR and apo-RAR in the GST pull-down assay. (C) Heterodimerization with TR alters protease sensitivity of RXR. Labeled RXR was incubated with a fivefold excess of unlabeled TR (in the presence and absence of T3 [12.5 μM]), then exposed to trypsin (1 mg/ml) for 3 min. (D) Model of conclusions from panels A, B, and C. (E and F) Gal4-RXRΔ449A7 cannot rescue Gal4-TR(AHT) for repression (E) and CoR interaction (F). (G) H12-AF2 mutants of TR used in panel H. (H) Ligand binding by TR abolishes complementation of repression between Gal4-RXR and Gal4-TR(AHT)E403A but not Gal4-TR(AHT)ΔAF2. Gal4-TR(AHT)ΔAF2, Gal4-TR(AHT)E403A, and Gal4-RXR were transfected separately or together and assayed for repression of (Gal4 × 5)-simian virus 40-luciferase reporter in the presence and absence of T3 (1 μM).