Abstract
The role of the local tumour and stromal immune landscape is increasingly recognised to be important in cancer development, progression and response to therapy. The composition, function, spatial orientation and gene expression profile of the infiltrate of the innate and adaptive immune system at the tumour and surrounding tissue has an established prognostic role in colorectal cancer (CRC). Multiple studies have confirmed that a tumour immune microenvironment (TIME) reflective of a type 1 adaptive immune response is associated with improved prognosis. There have been significant efforts to evolve these observations into validated, histopathology-based prognostic biomarkers, such as the Immunoscore. However, the clinical need lies much more in the development of predictive, not prognostic, biomarkers which have the potential to improve patient outcomes. This is particularly pertinent to help guide cytotoxic chemotherapy use in CRC, which remains the standard of care. Cytotoxic chemotherapy has recognised immunomodulatory activity distinct from its antimitotic effects, including mechanisms such as immunogenic cell death (ICD) and induction/inhibition of key immune players. Response to chemotherapy may differ with regard to molecular subtype of CRC, which are strongly associated with immune phenotypes. Thus, immune markers are potentially useful, though under-reported, predictive biomarkers. In this review, we discuss the impact of the TIME on response to cytotoxic chemotherapy in CRC, with a focus on baseline immune markers, and associated genomic and transcriptomic signatures.
Keywords: colorectal neoplasms, biomarkers, tumour, immunohistochemistry, stromal cells, lymphocytes
Introduction
The tumour immune microenvironment (TIME) has an important role in mediating cytotoxic drug response and resistance, as illustrated by the differences in efficacy between in vitro, ectopic tumour mouse models and humans.1 The TIME is extremely complex in colorectal cancer (CRC), reflecting genomic, host immunity and environmental (including microbiome) diversity.2 The immune visibility and susceptibility of CRCs can vary widely, and explain differential prognosis. The baseline TIME may facilitate immune evasion through low antigenicity, paucity of immune effectors or immunosuppressive mechanisms, which may contribute to primary resistance to chemotherapy. However, it is hypothesised that immunostimulatory chemotherapy may overcome these deficits specifically to improve prognosis, or conversely be redundant in an optimally infiltrated tumour. There is a significant clinical need to identify biomarkers of response to the standard cytotoxics used in CRC—the antimetabolites (5-fluorouracil (5-FU) and capecitabine), platinum derivatives (oxaliplatin) and topoisomerase inhibitors (irinotecan). This review will summarise the key literature and studies that focus on baseline, pretreatment TIME histopathological markers as potential predictive and prognostic biomarkers in patients with CRC receiving cytotoxic chemotherapy. Biomarkers relevant to radiotherapy and novel immunotherapies are outside the scope of this review.
Time assessment in CRC
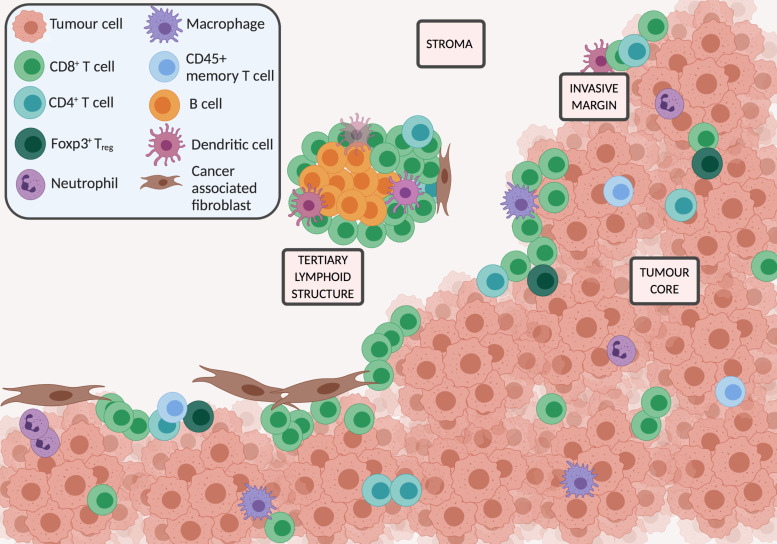
The TIME is composed of various infiltrating cells of the innate and adaptive immune system and their associated mediators. Immune cells can be identified in the core of the tumour (CT), both in intraepithelial cancer cell nests, or the tumour stroma (CS); at the invasive margin (IM), and in organised tertiary lymphoid structures (TLS) distant from the tumour3 (figure 1). This nomenclature will be used in the review to identify biomarker location where identified in respective papers. The cell type, location, density and functional orientation are all relevant for prognostication. Peritumoural infiltrates can be assessed on H&E-stained slides, using semiquantitative validated scoring systems including the Klintrup-Mäkinen (KM) grade4 and the Jass score.5 Multiplex immunohistochemical (IHC) techniques in clinically annotated tumour slides, to identify specific immune cells based on surface markers, is currently one of the key assessments of the TIME. Whole slides can be assessed, or tissue microarray techniques used to allow high throughput of samples. Cell density estimation can be performed manually, or assessed through digital image analysis6 and machine learning algorithms to allow objective quantification, although scoring methodology varies widely. Advances in RNA sequencing, proteomics and single-cell technologies are also increasingly used to assess the TIME. Techniques such as CIBERSOrT7 and MCP-counter8 can estimate the abundance of immune infiltrate in the tumour using the gene expression data from bulk tissues. Mass cytometry provides data at the individual cell level, and single-cell RNA sequencing allows profiling and classification of individual immune cells.9 Tumour heterogeneity and sampling issues add complexity to the use of biopsy-driven TIME biomarkers. Key cell types analysed using IHC techniques are listed in table 1, in addition to a summary of their known prognostic and predictive associations.
Figure 1.
Key cells and locations in the tumour immune microenvironment.
Table 1.
Primary tumour prognostic and predictive IHC-based TIME biomarkers in patients receiving chemotherapy
| Immune biomarker | Location | Prognostic role in early stage patients receiving adjuvant chemotherapy (regime) | Prognostic role in stage IV patients receiving palliative chemotherapy (regime) | Predictive role or differential biomarker prognostic role by treatment group |
| Specific immune cell | ||||
| CD3+ | CT | Most studies - î density=positive prognostic assoc
|
î density=positive prognostic assoc
|
Possible negative predictive role (adjuvant chemotherapy unspecified)
|
| IM | î density=mixed findings | î density=positive prognostic assoc
|
||
| CD8+ | CT (CS) | Most studies - î density=positive prognostic assoc | î density=mixed findings | No predictive role (adjuvant 5-FU)
|
| IM | Most studies - î density=positive prognostic assoc | No prognostic assoc
|
Possible positive predictive role (adjuvant 5-FU)
|
|
| CD4+ | CT | î density=mixed findings | ||
| IM | î density=positive prognostic assoc
|
|||
| Immunoscore (CD3+ and CD8+ CT + IM) |
0–4 | High score=positive prognostic assoc | Positive predictive role stage III (various adjuvant regimes)
Not predictive stage II (adjuvant 5-FU)65 |
|
| Foxp3+ (Treg) | CT | î density=mixed findings
|
î density=mixed findings | Mixed findings |
| IM | No prognostic association
|
No prognostic association
|
Possible negative predictive role (adjuvant chemotherapy unspecified)
|
|
| CD66b+ (TAN) | CT | î density=positive prognostic association | Mixed findings
|
|
| IM | î density=positive prognostic association
|
Possible positive predictive role (adjuvant 5-FU)
|
||
| CD68+ (general TAM marker) | CT | No prognostic assoc stage II
|
î density=negative prognostic association
|
|
| IM | î density=positive prognostic association
|
No prognostic association
|
Possible positive predictive role (adjuvant 5-FU)
|
|
| CD163+ (M2 polarised TAM) | CT | î density=negative prognostic association
|
î density=negative prognostic association
|
Possible negative predictive role (unspecified adjuvant regime)
|
| IM | No prognostic association
|
|||
| CD206+ (M2 polarised TAM) | CT | î density=negative prognostic association
|
Possible positive predictive role (adjuvant 5-FU)
|
|
| CD45RO+ (memory T cell) | CT | î density=positive prognostic association
|
î density=positive prognostic association
|
|
| IM | î density=positive prognostic association
|
No predictive role (adjuvant chemotherapy unspecified)
|
||
CS, core tumour stroma; CT, core tumour; DFS, disease-free survival; 5-FU, 5-fluorouracil; IHC, immunohistochemical; IM, invasive margin; OS, overall survival; TAM, tumour-associated macrophage; TAN, tumour-associated neutrophil; TIME, tumour immune microenvironment; TLS, tertiary lymphoid structures.
Immunomodulatory mechanism of action of cytotoxic chemotherapy
Many chemotherapeutic agents, including oxaliplatin, fluoropyrimidines and irinotecan, have local and systemic immunomodulatory effects beyond their cytostatic mechanisms.10–12 Preclinical models demonstrate that chemotherapy can augment immune responses directly by activation of immune effector cells (eg, production of interferon (IFN)γ) or inhibition of immunosuppressive factors (such as circulating regulatory T cells (Tregs)13), or act on tumours directly to increase antigenicity,14 15 immunogenicity15 or susceptibility to immune attack through other mechanisms.12 16 A small repertoire of chemotherapeutics, including oxaliplatin, can generate a specific mechanism of cell death, termed ‘immunogenic cell death’ (ICD), whereby release of specific danger signals from dying tumour cells stimulates a dendritic cell (DC)-mediated, cytotoxic T-helper 1 (Th1) response to eradicate residual tumour cells.17–19 Platinum cytotoxics can cause DC maturation,14 downregulate immune checkpoints and thus increase CD8+ T cell activation.20 21 In vivo, fluoropyrimidines selectively deplete immunosuppressive myeloid-derived suppressor cells (MDSCs),22 although have also been associated with a pro-tumour Th17 response.23 24 The immunogenicity of irinotecan is less certain, although in vivo work has reported influence on Treg and MDSC infiltration,25 and upregulation of tumour PD-L1.26 For clinical correlation, patients receiving neoadjuvant (preoperative) 5-FU/oxaliplatin show increased infiltration of CD3+,27 28 natural killer (NK) and CD8+ cells29 in resected liver metastases compared with patients undergoing upfront surgery. Neoadjuvant fluoropyrimidines increase the density of CD3+ CS and CD8+ CS cells in patients with resected rectal cancer compared with pretreatment biopsies.30 31
TIME BIOMARKERS
Inflammatory infiltrate
Increased tumour inflammatory infiltrate is strongly associated with improved survival,32 although most studies do not specify survival by subgroups based on chemotherapy utilisation. For those studies that do, an increased infiltrate seems to confer a positive prognostic advantage in patients receiving chemotherapy, mirroring the trend in the untreated population. A higher KM grade (more florid infiltrate at invasive margin) is associated with improved overall survival (OS) in patients receiving adjuvant chemotherapy (unspecified regimes)33 34 and FOLFOX (infusional 5-FU and oxaliplatin) chemotherapy.35 36 Tumour-infiltrating lymphocyte (TIL) density CT and IM was not prognostic in stage II/III patients receiving adjuvant 5-FU plus oxaliplatin regimes37; however, increased primary TIL density was associated with improved response rates (79% vs 48%, p=0.025) to doublet chemotherapy (oxaliplatin or irinotecan based) in patients with metastatic disease.38 This is notable as the primary tumour TIME appeared to impact on response rates at distant metastatic sites. Morris et al 39 reported a significant survival benefit with adjuvant 5-FU chemotherapy versus observation in stage III patients (n=1156) with peritumoural TILs present (HR 0.22, p<0.001) which was not evident in patients with absent TILs (HR 0.84, p=0.29). This suggests a possible predictive role, with 5-FU being more efficacious in patients with pre-existing immune recognition; however, non-standardised methods were used to identify TILs in this study which may impact validity.
CD3+/CD8+ T cells
The predominant infiltrating immune cells in CRC are T lymphocytes, identified by the generic CD3+ surface marker. Cytotoxic CD8+ T lymphocytes recognise tumour antigen presented by MHC class I molecules, thus providing the key antitumour immune response. High density of CD3+ and CD8+ T cells in the core tumour and invasive margin are well established as a positive prognostic marker in the majority of CRC studies.32 However, location is relevant—tumours demonstrating a paucity of CD8+ cells in the tumour core, and lacking the activation markers granzyme-B and IFNγ, have been termed ‘infiltrated excluded’ with worse survival outcomes.1 The prognostic associations in chemotherapy-treated patients are less well reported. Retrospective studies have confirmed a positive survival association of increased density CD3+ CT in patients receiving single agent 5-FU40–43 and FOLFOX,44 45 although some groups have found no relationship.46 47 CD3+ IM was not prognostic for single agent 5-FU chemotherapy,40 48 and this may reflect the phenomenon of the ‘infiltrated excluded’ tumour discussed above, which could impact on 5-FU efficacy. In contrast, increased density of CD3+ IM did correlate with improved disease-free survival (DFS) in a large prospective phase III trials of patients receiving adjuvant FOLFOX±cetuximab (an epidermal growth factor receptor monoclonal antibody).44 45 49 It is possible that the addition of oxaliplatin to 5-FU may influence the prognostic impact of invasive margin T cells. Increased density of CD8+ CS was positively prognostic in patients with early stage disease receiving adjuvant 5-FU single agent chemotherapy,42 46 50 51 ±bevacizumab,47 and high CD8+ CT/CS and IM was associated with improved DFS in patients receiving oxaliplatin doublet adjuvant chemotherapy.44 45 52 Some studies have reported that the relative survival benefit of adjuvant 5-FU chemotherapy is much greater for patients with increased density of CD8+ CT compared with patients with low density,53 supporting Morris et al’s findings,39 and suggesting fluoropyrimidines may be more efficacious when a pre-existing Th1 response is present. However, a treatment interaction has not been confirmed by other groups.46 CD8+ CT as a prognostic marker in stage IV patients has shown contradictory results (see table 1).54–56 Multiple studies30 57 58 have correlated high pretreatment CD3+ and CD8+ cell density on rectal biopsy with increased response rates to neoadjuvant therapy and improved survival, although this has not been replicated in all reports,31 59 and outcomes are mediated by the effects of radiotherapy and are thus outside the scope of this review.
Immunoscore
The Immunoscore (IS) was designed as a digitally quantified IHC assessment of CD8+ CT + IM and memory T cell (CD45RO+ CT+IM) densities added to produce a cumulative score.60 It has been validated to show prognostic ability superior to the traditional tumour/node/metastasis (TNM) staging system,61 with high scores conferring superior survival. CD3+ later replaced CD45RO+ due to superior antibody performance3 (figure 2). Its validity as a prognostic marker in patients receiving adjuvant 5-FU33 62 and FOLFOX45 63 chemotherapy has been reported, but its role as a predictive marker is less clear. In a recent multinational trial of stage III patients, those with a low IS (0–1) did not benefit from adjuvant chemotherapy (various regimes), whereas those with IS 2–4 did, and the magnitude of the survival benefit was greater the higher the IS.64 In high-risk stage II disease, high IS was prognostic, but not a predictive discriminator of 5-FU benefit.65 Interestingly, in an analysis of stage III patients in the IDEA collaboration (3 months vs 6 months of adjuvant FOLFOX), patients with IS 2–4 had a significantly improved DFS with 6 months vs 3 months of FOLFOX (HR 0.53, p=0.0003), whereas patients with IS 0–1 did not derive a significant DFS benefit with extended treatment (HR 0.84, p=0.27).63 This suggests again possible futility of doublet regimes, irrespective of cumulative dose, in immune-excluded disease and a dose-dependent benefit of oxaliplatin regimes in tumours with a baseline cytotoxic T lymphocyte response.
Figure 2.
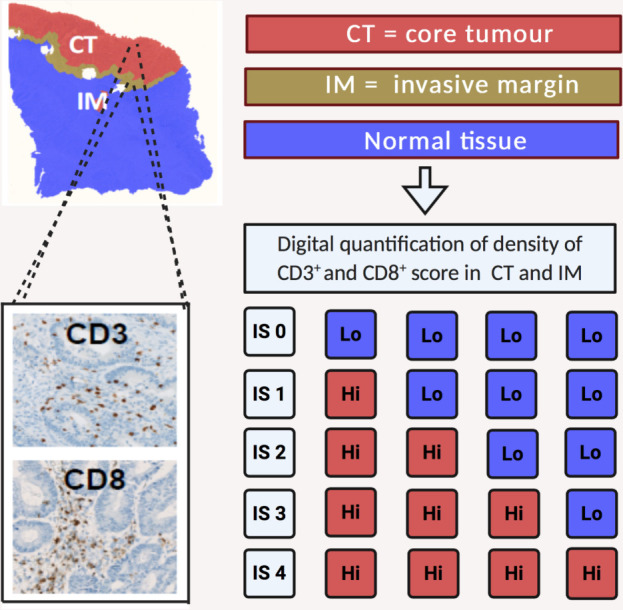
The Immunoscore (IS) is based on the numeration of two lymphocyte populations (CD3+ and CD8+) in the CT and IM. Density of cells is determined using an image analysis workstation. Each marker in a specified region is categorised as ‘Hi’ or ‘Lo’ based on predetermined cut-off values. Patients are stratified according to a score IS 0 to IS 4 based on the total number of ‘Hi’ densities observed in the four regions.
CD4+/Foxp3+ T cells
CD4+ helper T cells, which aid tumour immune responses by activation of signalling to facilitate CD8+ T cell-mediated cell death, can exert both Th1 responses which promote antitumour effects with good prognostic association,66 and Th2 responses which are tumourigenic. CD4+ CT as a prognostic marker has shown positive association in only half of the studies it has been assessed, and in no studies of CD4+ IM.32 Adjuvant studies referencing chemotherapy are lacking. Increased primary tumour CD4+ CS and IM was prognostic in some studies of stage IV patients receiving mixed palliative regimes55 but not in other cohorts receiving oxaliplatin regimes.56 Tregs constitute a specific subtype of CD4+ T cell, identified by immunoprofile CD25+Foxp3+, and have general immunosuppressive functions, although this can vary depending on marker expression.67 Meta-analyses of prognostic studies in CRC has reported a positive association with cancer-specific survival68 (CSS) and OS69 which is in contrast to other tumour types. High density of Foxp3+ CT/CS has been associated with improved OS in some cohorts receiving adjuvant 5-FU chemotherapy,40 42 46 50 and palliative oxaliplatin,56 but not others.41 70 71 Some cohorts have suggested that increased Foxp3+ density may confer a positive prognostic association only in untreated patients, and not in patients receiving chemotherapy,70 71 although true predictive studies are required.
Tumour-associated neutrophil
Tumour-associated neutrophils (TANs), identified by their markers CD11b+, CD66+ and Ly6G+, are less populous than other cells, and subsets can be either tumour-suppressive or supportive depending on TGF-β and IFN-γ signalling.72 Prognostic studies have reported conflicting results. In stage I-III patients (n=1008), high TAN (CD66+ CT) density conferred an excellent prognosis, and no benefit from adjuvant chemotherapy, whereas low density conferred worse prognosis and poorer survival in patients receiving adjuvant chemotherapy.70 However, this unexpected result may be a reflection of treatment bias and lack of adjustment for tumour stage and necrosis, which are associated with TAN density.73 In a contradictory smaller cohort of stage III patients, high TANCT density was reported as a negative prognostic marker in patients undergoing surgery (DFS HR 3.0, p=0.07). However, this impact was mitigated by the use of adjuvant 5-FU, whereby patients with high TANCT had improved prognosis.74 High CD66b+ IM was also a positive prognostic and predictive marker in stage III patients receiving adjuvant 5-FU.75 Contradictory results may also be explained in this and other studies by variations in methodology, including different prognostic associations depending on assessment in invasive margin or core tumour,73 and variable marker categorisation.
Tumour-associated macrophages
Tumour-associated macrophages (TAMs), often identified by the non-specific CD68+ monocyte lineage marker, are broadly grouped into two phenotypes. The classically activated (M1) type (surface markers iNOS, CD86+, CD169+) that stimulate antitumour immune responses, and the alternatively activated (M2) type (surface markers CD163+, CD206+, CD204+) that enhance tumour progression and suppress immune response (eg, NK and T cell mediated killing).76 Increased CD68+ IM density was a positive predictive marker of 5-FU benefit in a small cohort, and in a companion in vitro study, 5-FU and M1-macrophages showed synergistic impact on cell death in CRC cell lines.75 M2CT infiltration has been reported as negatively prognostic in several studies of patients treated with systemic chemotherapy.55 70 77 78 In vitro work has suggested that M2 macrophages confer resistance to 5-FU,79 and some studies suggest a negative predictive relationship.70 Feng et al 77 reported high CD206+:CD68+ ratio (increased proportion of M2 macrophages) was a marker for poorer DFS and OS in stage II disease, although also predicted a significant survival benefit from adjuvant 5-FU-based chemotherapy versus observation (DFS HR 0.42, p=0.003) which was not present in the better prognostic group with a low ratio (HR 0.99, p=0.99). Oxaliplatin plus trifluridine/tipiracil (an antimetabolite) depletes M2 macrophages, resulting in higher CD8+ infiltration and better therapeutic efficacy.80 Further exploration in patient cohorts using differential chemotherapy regimes is required.
CD45RO+ T cells
Central and effector memory T cells (characterised by CD45RO+ marker) drive secondary immune responses post exposure to primary antigens. Meta-analyses suggest a positive prognostic association for increased density of these cells both in the core tumour and invasive margin.32 High density of primary tumour CD45RO+ CT 42 43 and CD45RO+ IM 71 is an independent prognostic factor for improved OS in early stage disease patients receiving 5-FU. High density was associated with better survival in patients with stage IV CRC undergoing adjuvant oxaliplatin or irinotecan chemotherapy post-curative intent resection (estimated 3-year survival 62% vs 27%, p=0.007).54 High CCR7+ CS (used to identify CD8+ naïve and central memory T cells) was associated with improved OS in patients receiving palliative oxaliplatin-based regimes.81
Gamma delta (γδ) T cells
Gamma delta T cells are a rare subset of predominantly mucosal CD8-CD4- T cells with a broad functional role in cytokine (IFN-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-17) and chemokine (RANTES, IP-10, lymphotactin) production, cytolysis and coordination of antigen presentation.82 In vivo studies show that ICD-inducing chemotherapy causes a rapid invasion of γδ T lymphocytes prior to the invasion of CD8+ T cells, and that in TCR δ-/- mice, the therapeutic efficacy of chemotherapy was reduced.83 Increased expression of γδ T cells has been associated with improved DFS in patients with CRC.84 While results from CRC cohorts receiving chemotherapy are under-reported, a series (n=463) of patients with gastric cancer receiving adjuvant 5-FU chemotherapy suggest a significant survival advantage of chemotherapy versus observation if infiltrating γδ T cells were increased.85
B cells
B cells also recognise tumour antigens, produce tumour-specific antibodies and are identified through CD19+, CD20+ and CD78+ markers. High CD20+ CS has been associated with better prognosis in CRC,86 as has the presence of TLSs, which contain concentrated B cells.87 However, CD20+ CT or IM was not prognostic in patients receiving adjuvant FOLFOX.45
Immune checkpoints
Multiple stimulatory and inhibitory immune checkpoints, crucial for self-tolerance, and co-opted by tumours to evade immunosurveillance, have been identified in the TIME. One such checkpoint, programmed death-ligand 1 (PD-L1), is predominantly derived from the immune infiltrate,88 not tumour cells, in CRC. Immunodeficient murine xenograft models of PD-L1 knockout tumours display resistance to oxaliplatin,89 which contrasts with models in other tumour types. In early stage patients receiving 5-FU chemotherapy, high tumour PD-L1 was not prognostic in some studies,40 42 although negatively impacted on DFS in another stage III cohort receiving adjuvant chemotherapy.90 In contrast, PD-L1 expression on immune infiltrating mononuclear cells was associated with longer DFS. Dunne et al 91 reported that in stage III CRC (n=201), PD-L1low tumours conferred a significant DFS benefit from adjuvant chemotherapy versus observation (adjusted HR 0.44, p=0.0062), and the use of adjuvant chemotherapy was able to overcome the negative prognostic impact of low PD-L1. However, in contrast, PD-L1high expression resulted in inferior DFS post adjuvant chemotherapy versus observation (unadjusted HR 4.95, 95% CI 1.10 to 22.35, p=0.02), although the significance was lost on multivariate analysis. This is one of the first series to suggest a possible detrimental effect of chemotherapy in tumours which overexpress PD-L1.
Immune markers associated with microsatellite instability
Tumours harbouring microsatellite instability (MSI) have defects in the DNA mismatch repair system (dMMR), and thus display a hypermutable phenotype. The differential improved survival in patients with early stage dMMR tumours has been extensively reported and is partly attributable to increased immune stimulation in these tumours due to the increased neoantigen load. MSI tumours have a dense infiltration of CD8+ cells,92 a Th1 cytokine response, and also overexpress many inhibitory immune checkpoints (including PD-1, PD-L1, CTLA-4, LAG-3 and IDO).93 However, MSI tumours are more chemoresistant to 5-FU than microsatellite stable (MSS) lines in preclinical models.94 The relative survival benefit from FOLFOX compared with 5-FU is much greater in stage II-III dMMR patients compared with pMMR,95 suggesting possible resistance to 5-FU alone. However, in a recent report from the FoXTROT trial, dMMR colon cancers showed significantly reduced pathological response rates to neoadjuvant oxaliplatin doublet chemotherapy than pMMR,96 and clinical progression through this chemotherapy regime was more common in dMMR than pMMR rectal cancers (29% vs 0%, p=0.0001).97 In contrast, in the metastatic setting, MSI status did not affect response rates to palliative FOLFOX chemotherapy.98 Regarding irinotecan therapy, in both cell lines and tumour xenografts, dMMR tumours are more sensitive to irinotecan than MMR proficient (pMMR) lines.99 100 In a small retrospective cohort, response rates to palliative 5-FU plus irinotecan were much higher in MSI than MSS disease (57% vs 10%, p=0.009),101 and DFS was longer in MSI tumours receiving an irinotecan containing regime in a separate cohort.102 In a large adjuvant phase III trial, only patients with dMMR tumours received a DFS benefit from adding irinotecan to 5-FU,103 but this MSI/treatment interaction was not confirmed in another similar trial.104 The relevance of immunological variation on chemotherapeutic response in the context of genetic alterations is largely unknown.
Immunogenic Cell Death markers
ICD is the cornerstone of the immunomodulatory action of oxaliplatin and associated markers are potential predictive biomarkers (figure 3). DC activation is a key step in ICD. However, identification of DCs, which show functional diversity and heterogeneous activation states, can be challenging and markers are variably reported between studies and may account for conflicting results reporting both good105–109 and bad110 prognostic association. In vivo studies have demonstrated that blockade of surface calreticulin exposure111 and HMGB1-dependent TLR-4 signalling,112 both key steps in ICD, severely compromised the cytotoxicity of oxaliplatin chemotherapy. Stromal calreticulin expression is associated with infiltration of CD45RO+ cells and improved OS in univariate analysis in patients receiving adjuvant 5-FU.113
Figure 3.
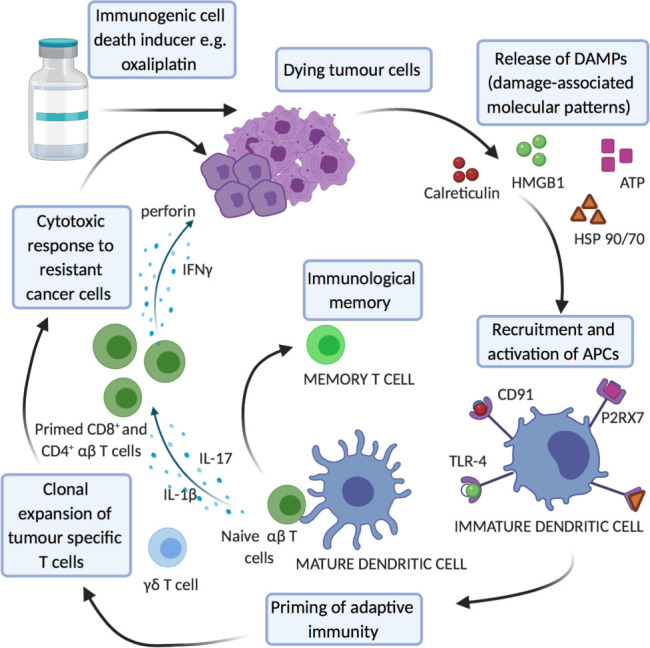
Key cells and pathways in immunogenic cell death as potential predictive biomarkers. IFNγ, interferon; IL, interleukin.
Stromal markers
The tumour stroma plays a direct and indirect role in modulating response to immunomodulatory chemotherapy. De novo drug resistance may occur from environment-mediated phenomena, where cancer cells are protected from treatment-induced apoptosis by ‘barriers’, including either soluble secreted factors or cell-adhesion-mediated mechanisms.114 The tumour:stroma percentage is a validated prognostic marker, with increased stromal percentage associated with poorer prognosis, including in chemotherapy-treated patients.115 Cancer-associated fibroblasts (CAFs) are a heterogeneous group of fibroblast-like cells that release certain cytokines, growth factors and proinflammatory factors. In vitro cell line studies suggest CAFs trigger a JAK/STAT pathway signalling cascade that leads to reduced response rates to oxaliplatin and 5-FU,116 and stromal CAF-derived conditioned medium primed the growth of cancer stem cells after treatment with 5-FU and oxaliplatin, thus increasing their inherent chemoresistance.117 High CAF infiltration is associated with worse DFS in adjuvant-treated patients118 and associated-induced expression of their surrogate markers smooth muscle actin and survivin have been related to worse survival in 5-FU119 and oxaliplatin-treated advanced patients.120
Genomic markers and transcriptomic profiles
Recent advances in high-throughput gene testing technology have led to the development of some molecular signatures for chemotherapy prediction. Increased expression of infiltrating immune cells, as identified by CIBERSOrT, showed a trend to improved overall survival in patients receiving chemotherapy.121 Multiple classifications of CRC, based on molecular transcriptomic data, have been proposed in recent years, and unified into the Consensus Molecular Subtypes (CMS). This incorporates gene expression profiles from the tumour, stroma and immune cells to differentiate four groups (CMS1-4) and are highly correlated with immune cell infiltration patterns.122 The CMS1 subgroup (MSI-like) is enriched for genes coding for CD8+ and CD68+ cells, T-cell attracting chemokines, TLSs and Th1 cytokines. The CMS4 subgroup (mesenchymal) is enriched for expression of genes encoding CD8+ cells, MDSCs, Tregs, Th17+ cells, angiogenic factors and immunosuppressive molecules (eg, TGFβ1). Both CMS2 (canonical) and CMS3 (metabolic) subgroups exhibit low-immune and low-inflammatory signatures. In a retrospective taxonomy study, only CMS2 and 3 subgroups derived a benefit from adjuvant chemotherapy (unspecified) in stage III disease, with CMS4 showing a trend to benefit.123 Song et al 124 used an alternative transcriptomic classifier (CRCA) to examine patients in the NSABP-07 trial (adjuvant FOLFOX vs 5-FU), and reported only patients with an ‘enterocyte’ subtype (with immune features similar to the ‘cold’ CMS2) derived a benefit from the addition of oxaliplatin, with a significant interaction test. The same group repeated the analysis using patients enrolled on the MOSAIQ trial (adjuvant CAPOX vs capecitabine) but did not find any association,125 which may be due to different fluoropyrimidine use or oxaliplatin schedule, which have shown different interactions in other immune biomarker studies.36 CMS1 patients have worse OS with FOLFIRI-based regimes compared with the other CMS subtypes in the FIRE-3 trial126; however, they also show improved OS with the addition of bevacizumab in the metastatic setting.127 Published studies suggest a trend to 5FU/oxaliplatin resistance in CMS4 (or similar classifier) patients, both in the adjuvant127 128 and metastatic setting,129 where first-line irinotecan regimes showed better response rates and survival.130 131
Immune infiltrate in resected metastases
Several reports have assessed the prognostic and predictive impact of the TIME from resected metastases, predominantly liver metastases,132 which appears to correlate with the primary tumour. However, many of the studies include patients receiving neoadjuvant therapy, which can alter the immune infiltrate substantially. Metastatic disease has a different immunological milieu which is defined by tumour immune evasion. Liver metastases with pretreatment high Immunoscore (and high CD3+, CD8+, and CD20+ cells133) are associated with increased response to chemotherapy (p=0.009) and improved DFS and OS.55 The type of postoperative chemotherapy/adjunct did not impact survival. However, a high IS is not tantamount to excellent prognosis in this setting (as opposed to with early stage disease) as most patients relapsed after surgery. The authors showed the density of CD8+:CD20+ CT+IM to be an additional strong prognostic discriminator. A high ‘density score’ (based on a cumulative density of CD3+, CD8+ and granzyme B in liver metastases) was also reported by Halama et al 134 to have significant prognostic ability in stage IV patients receiving any regime of chemotherapy (HR OS 0.06, p<0.01).
Conclusions
Here, we have reviewed CRC studies focusing on the TIME and found that the prognostic ability of these markers in CRC is mediated in the context of chemotherapy, and true predictive studies are under-reported. While prognostic biomarkers have been used as a surrogate for predictive markers, with an assumption that patients with ‘poor’ prognosis will gain a greater absolute benefit from chemotherapy, this may be untrue, especially if the biomarker is also a marker of therapy resistance. Nevertheless, current reports indicate that the relative benefit of 5-FU chemotherapy may be enhanced in the context of some pre-existing CD8+/CD3+ infiltration in core tumour, but may be unnecessary or importantly even detrimental in the milieu of a highly inflamed TIME. CRCs with high immunosuppressive pathways may also be more resistant to oxaliplatin doublets. Furthermore, chemotherapy may improve prognosis in cancers driven by specific immune cell populations, such as TAMs and TANs. The emerging move to standardise assessment of TILs/IHC-based markers in CRC reporting has the potential for more robust prospective trials. Such trials are needed to develop better clinical biomarkers for therapy benefit and cytotoxic effects of chemotherapy. Patients likely having adverse effects may require de-escalated or even no therapy, and some may require alternative or combination agents, which have shown early promise.135 Significant research and development is in progress with regard to such adjuncts, which include various combination approaches with synergistic benefits136 and novel immunotherapies, to improve precision medicine in the future.
Take home messages.
The tumour immune microenvironment has an important role to play in mediating cytotoxic chemotherapy response and primary resistance.
Many chemotherapy agents used in colorectal cancer have local and systemic immunomodulatory effects.
Baseline tumour immune cells, including T cell subsets, tumour-associated neutrophils and macrophages, may represent potential predictive biomarker predicting response and resistance to cytotoxic chemotherapy.
Prospective trials using standardised validated markers, such as the Immunoscore, are required to rationalise the use of adjuvant chemotherapy and target different palliative chemotherapy regimes and adjuncts to patients more likely to respond.
Footnotes
Handling editor: Runjan Chetty.
Contributors: All authors have contributed to the production of this manuscript. Images created with Biorender.com.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (time) for effective therapy. Nat Med 2018;24:541–50. 10.1038/s41591-018-0014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews MC, Reuben A, Gopalakrishnan V, et al. Concepts collide: genomic, immune, and microbial influences on the tumor microenvironment and response to cancer therapy. Front Immunol 2018;9:946. 10.3389/fimmu.2018.00946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol 2014;232:199–209. 10.1002/path.4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klintrup K, Mäkinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer 2005;41:2645–54. 10.1016/j.ejca.2005.07.017 [DOI] [PubMed] [Google Scholar]
- 5.Jass JR. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol 1986;39:585–9. 10.1136/jcp.39.6.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolte S, Zlobec I, Lugli A, et al. Construction and analysis of tissue microarrays in the era of digital pathology: a pilot study targeting Cdx1 and CDX2 in a colon cancer cohort of 612 patients. J Pathol Clin Res 2017;3:58–70. 10.1002/cjp2.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015;12:453–7. 10.1038/nmeth.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becht E, Giraldo NA, Lacroix L, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol 2016;17:218. 10.1186/s13059-016-1070-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang B, Lee JH, Bang D. Single-Cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med 2018;50:96. 10.1038/s12276-018-0071-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bracci L, Schiavoni G, Sistigu A, et al. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 2014;21:15–25. 10.1038/cdd.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galluzzi L, Buqué A, Kepp O, et al. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015;28:690–714. 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 12.Zitvogel L, Galluzzi L, Smyth MJ, et al. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013;39:74–88. 10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 13.Maeda K, Hazama S, Tokuno K, et al. Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res 2011;31:4569–74. [PubMed] [Google Scholar]
- 14.Liu WM, Fowler DW, Smith P, et al. Pre-Treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer 2010;102:115–23. 10.1038/sj.bjc.6605465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohtsukasa S, Okabe S, Yamashita H, et al. Increased expression of CEA and MHC class I in colorectal cancer cell lines exposed to chemotherapy drugs. J Cancer Res Clin Oncol 2003;129:719–26. 10.1007/s00432-003-0492-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesterhuis WJ, Punt CJA, Hato SV, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest 2011;121:3100–8. 10.1172/JCI43656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005;202:1691–701. 10.1084/jem.20050915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010;29:482–91. 10.1038/onc.2009.356 [DOI] [PubMed] [Google Scholar]
- 19.Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51–72. 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 20.Hato SV, Khong A, de Vries IJM, et al. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res 2014;20:2831–7. 10.1158/1078-0432.CCR-13-3141 [DOI] [PubMed] [Google Scholar]
- 21.Guan Y, Kraus SG, Quaney MJ, et al. Folfox chemotherapy ameliorates CD8 T lymphocyte exhaustion and enhances checkpoint blockade efficacy in colorectal cancer. Front Oncol 2020;10:586. 10.3389/fonc.2020.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010;70:3052–61. 10.1158/0008-5472.CAN-09-3690 [DOI] [PubMed] [Google Scholar]
- 23.Ghiringhelli F, Bruchard M, Apetoh L. Immune effects of 5-fluorouracil: ambivalence matters. Oncoimmunology 2013;2:e23139. 10.4161/onci.23139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruchard M, Mignot G, Derangère V, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the NLRP3 inflammasome and promotes tumor growth. Nat Med 2013;19:57–64. 10.1038/nm.2999 [DOI] [PubMed] [Google Scholar]
- 25.Kanterman J, Sade-Feldman M, Biton M, et al. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res 2014;74:6022–35. 10.1158/0008-5472.CAN-14-0657 [DOI] [PubMed] [Google Scholar]
- 26.Iwai T, Sugimoto M, Wakita D, et al. Topoisomerase I inhibitor, irinotecan, depletes regulatory T cells and up-regulates MHC class I and PD-L1 expression, resulting in a supra-additive antitumor effect when combined with anti-PD-L1 antibodies. Oncotarget 2018;9:31411–21. 10.18632/oncotarget.25830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanis E, Julié C, Emile J-F, et al. Prognostic impact of immune response in resectable colorectal liver metastases treated by surgery alone or surgery with perioperative FOLFOX in the randomised EORTC study 40983. Eur J Cancer 2015;51:2708–17. 10.1016/j.ejca.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 28.Donadon M, Hudspeth K, Cimino M, et al. Increased infiltration of natural killer and T cells in colorectal liver metastases improves patient overall survival. J Gastrointest Surg 2017;21:1226–36. 10.1007/s11605-017-3446-6 [DOI] [PubMed] [Google Scholar]
- 29.Ledys F, Klopfenstein Q, Truntzer C, et al. Ras status and neoadjuvant chemotherapy impact CD8+ cells and tumor HLA class I expression in liver metastatic colorectal cancer. J Immunother Cancer 2018;6:123. 10.1186/s40425-018-0438-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng F, Mu D, Meng X, et al. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am J Cancer Res 2015;5:2064–74. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, Bai W, Tong X, et al. Correlation between tumor microenvironment-associated factors and the efficacy and prognosis of neoadjuvant therapy for rectal cancer. Oncol Lett 2019;17:1062–70. 10.3892/ol.2018.9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander PG, McMillan DC, Park JH. The local inflammatory response in colorectal cancer - Type, location or density? A systematic review and meta-analysis. Cancer Treat Rev 2020;83:101949. 10.1016/j.ctrv.2019.101949 [DOI] [PubMed] [Google Scholar]
- 33.Park JH, McMillan DC, Edwards J, et al. Comparison of the prognostic value of measures of the tumor inflammatory cell infiltrate and tumor-associated stroma in patients with primary operable colorectal cancer. Oncoimmunology 2016;5:e1098801. 10.1080/2162402X.2015.1098801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hynes SO, Coleman HG, Kelly PJ, et al. Back to the future: routine morphological assessment of the tumour microenvironment is prognostic in stage II/III colon cancer in a large population-based study. Histopathology 2017;71:12–26. 10.1111/his.13181 [DOI] [PubMed] [Google Scholar]
- 35.Cha YJ, Park EJ, Baik SH, et al. Clinical significance of tumor-infiltrating lymphocytes and neutrophil-to-lymphocyte ratio in patients with stage III colon cancer who underwent surgery followed by FOLFOX chemotherapy. Sci Rep 2019;9:11617. 10.1038/s41598-019-48140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roseweir AK, Park JH, Hoorn ST, et al. Histological phenotypic subtypes predict recurrence risk and response to adjuvant chemotherapy in patients with stage III colorectal cancer. J Pathol Clin Res 2020;6:283–96. 10.1002/cjp2.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pentheroudakis G, Raptou G, Kotoula V, et al. Immune response gene expression in colorectal cancer carries distinct prognostic implications according to tissue, stage and site: a prospective retrospective translational study in the context of a Hellenic cooperative Oncology group randomised trial. PLoS One 2015;10:e0124612. 10.1371/journal.pone.0124612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibutani M, Maeda K, Nagahara H, et al. Tumor-Infiltrating lymphocytes predict the chemotherapeutic outcomes in patients with stage IV colorectal cancer. In Vivo 2018;32:151–8. 10.21873/invivo.11218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris M, Platell C, Iacopetta B. Tumor-Infiltrating lymphocytes and perforation in colon cancer predict positive response to 5-fluorouracil chemotherapy. Clin Cancer Res 2008;14:1413–7. 10.1158/1078-0432.CCR-07-1994 [DOI] [PubMed] [Google Scholar]
- 40.Miller TJ, McCoy MJ, Hemmings C, et al. The prognostic value of cancer stem-like cell markers Sox2 and CD133 in stage III colon cancer is modified by expression of the immune-related markers FOXP3, PD-L1 and CD3. Pathology 2017;49:721–30. 10.1016/j.pathol.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 41.Sinicrope FA, Rego RL, Ansell SM, et al. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 2009;137:1270–9. 10.1053/j.gastro.2009.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng Y, Li Y, Cai S, et al. Immunological nomograms predicting prognosis and guiding adjuvant chemotherapy in stage II colorectal cancer. Cancer Manag Res 2019;11:7279–94. 10.2147/CMAR.S212094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng R-Q, Wu X-J, Ding Y, et al. Co-expression of nuclear and cytoplasmic HMGB1 is inversely associated with infiltration of CD45RO+ T cells and prognosis in patients with stage IIIB colon cancer. BMC Cancer 2010;10:496. 10.1186/1471-2407-10-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reichling C, Taieb J, Derangere V, et al. Artificial intelligence-guided tissue analysis combined with immune infiltrate assessment predicts stage III colon cancer outcomes in PETACC08 study. Gut 2020;69:681–90. 10.1136/gutjnl-2019-319292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinicrope FA, Shi Q, Hermitte F, et al. Contribution of immunoscore and molecular features to survival prediction in stage III colon cancer. JNCI Cancer Spectr 2020;4:pkaa023. 10.1093/jncics/pkaa023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berntsson J, Svensson MC, Leandersson K, et al. The clinical impact of tumour-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: a cohort study. Int J Cancer 2017;141:1654–66. 10.1002/ijc.30869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glaire MA, Domingo E, Sveen A, et al. Tumour-infiltrating CD8+ lymphocytes and colorectal cancer recurrence by tumour and nodal stage. Br J Cancer 2019;121:474–82. 10.1038/s41416-019-0540-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laghi L, Bianchi P, Miranda E, et al. Cd3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol 2009;10:877–84. 10.1016/S1470-2045(09)70186-X [DOI] [PubMed] [Google Scholar]
- 49.Emile J-F, Julié C, Le Malicot K, et al. Prospective validation of a lymphocyte infiltration prognostic test in stage III colon cancer patients treated with adjuvant FOLFOX. Eur J Cancer 2017;82:16–24. 10.1016/j.ejca.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 50.Yoon HH, Orrock JM, Foster NR, et al. Prognostic impact of Foxp3+ regulatory T cells in relation to CD8+ T lymphocyte density in human colon carcinomas. PLoS One 2012;7:e42274. 10.1371/journal.pone.0042274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sperlich A, Balmert A, Doll D, et al. Genetic and immunological biomarkers predict metastatic disease recurrence in stage III colon cancer. BMC Cancer 2018;18:998. 10.1186/s12885-018-4940-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fountzilas E, Kotoula V, Tikas I, et al. Prognostic significance of tumor genotypes and CD8+ infiltrates in stage I-III colorectal cancer. Oncotarget 2018;9:35623–38. 10.18632/oncotarget.26256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prall F, Dührkop T, Weirich V, et al. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol 2004;35:808–16. 10.1016/j.humpath.2004.01.022 [DOI] [PubMed] [Google Scholar]
- 54.Lee W-S, Kang M, Baek J-H, et al. Clinical impact of tumor-infiltrating lymphocytes for survival in curatively resected stage IV colon cancer with isolated liver or lung metastasis. Ann Surg Oncol 2013;20:697–702. 10.1245/s10434-012-2752-1 [DOI] [PubMed] [Google Scholar]
- 55.Kwak Y, Koh J, Kim D-W, et al. Immunoscore encompassing CD3+ and CD8+ T cell densities in distant metastasis is a robust prognostic marker for advanced colorectal cancer. Oncotarget 2016;7:81778–90. 10.18632/oncotarget.13207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Correale P, Rotundo MS, Del Vecchio MT, et al. Regulatory (Foxp3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother 2010;33:435–41. 10.1097/CJI.0b013e3181d32f01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anitei M-G, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res 2014;20:1891–9. 10.1158/1078-0432.CCR-13-2830 [DOI] [PubMed] [Google Scholar]
- 58.Shinto E, Hase K, Hashiguchi Y, et al. Cd8+ and Foxp3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol 2014;21:414–21. 10.1245/s10434-014-3584-y [DOI] [PubMed] [Google Scholar]
- 59.McCoy MJ, Hemmings C, Anyaegbu CC, et al. Tumour-Infiltrating regulatory T cell density before neoadjuvant chemoradiotherapy for rectal cancer does not predict treatment response. Oncotarget 2017;8:19803–13. 10.18632/oncotarget.15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 61.Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009;27:5944–51. 10.1200/JCO.2008.19.6147 [DOI] [PubMed] [Google Scholar]
- 62.Wirta E-V, Seppälä T, Friman M, et al. Immunoscore in mismatch repair-proficient and -deficient colon cancer. J Pathol Clin Res 2017;3:203–13. 10.1002/cjp2.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pagès F, André T, Taieb J, et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol 2020;31:921–9. 10.1016/j.annonc.2020.03.310 [DOI] [PubMed] [Google Scholar]
- 64.Mlecnik B, Bifulco C, Bindea G, et al. Multicenter International Society for immunotherapy of cancer study of the consensus immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol 2020;38:3638–51. 10.1200/JCO.19.03205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galon J, Hermitte F, Mlecnik B, et al. Immunoscore clinical utility to identify good prognostic colon cancer stage II patients with high-risk clinico-pathological features for whom adjuvant treatment may be avoided [abstract]. J Clin Oncol 2019;37 (Supp 4):P487. 10.1200/JCO.2019.37.4_suppl.487 [DOI] [Google Scholar]
- 66.Ling A, Lundberg IV, Eklöf V, et al. The infiltration, and prognostic importance, of Th1 lymphocytes vary in molecular subgroups of colorectal cancer. J Pathol Clin Res 2016;2:21–31. 10.1002/cjp2.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saito T, Nishikawa H, Wada H, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med 2016;22:679–84. 10.1038/nm.4086 [DOI] [PubMed] [Google Scholar]
- 68.Xu P, Fan W, Zhang Z, et al. The clinicopathological and prognostic implications of FoxP3+regulatory T cells in patients with colorectal cancer: a meta-analysis. Front Physiol 2017;8:950. 10.3389/fphys.2017.00950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ling Z-A, Zhang L-J, Ye Z-H, et al. Immunohistochemical distribution of Foxp3+ regulatory T cells in colorectal cancer patients. Int J Clin Exp Pathol 2018;11:1841–54. [PMC free article] [PubMed] [Google Scholar]
- 70.Ye L, Zhang T, Kang Z, et al. Tumor-Infiltrating immune cells act as a marker for prognosis in colorectal cancer. Front Immunol 2019;10:2368. 10.3389/fimmu.2019.02368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim Y, Bae JM, Li G, et al. Image analyzer-based assessment of tumor-infiltrating T cell subsets and their prognostic values in colorectal carcinomas. PLoS One 2015;10:e0122183. 10.1371/journal.pone.0122183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mizuno R, Kawada K, Itatani Y, et al. The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci 2019;20:529. 10.3390/ijms20030529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wikberg ML, Ling A, Li X, et al. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum Pathol 2017;68:193–202. 10.1016/j.humpath.2017.08.028 [DOI] [PubMed] [Google Scholar]
- 74.Galdiero MR, Bianchi P, Grizzi F, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer 2016;139:446–56. 10.1002/ijc.30076 [DOI] [PubMed] [Google Scholar]
- 75.Malesci A, Bianchi P, Celesti G, et al. Tumor-associated macrophages and response to 5-fluorouracil adjuvant therapy in stage III colorectal cancer. Oncoimmunology 2017;6:e1342918. 10.1080/2162402X.2017.1342918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edin S, Wikberg ML, Dahlin AM, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One 2012;7:e47045. 10.1371/journal.pone.0047045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng Q, Chang W, Mao Y, et al. Tumor-Associated macrophages as prognostic and predictive biomarkers for postoperative adjuvant chemotherapy in patients with stage II colon cancer. Clin Cancer Res 2019;25:3896–907. 10.1158/1078-0432.CCR-18-2076 [DOI] [PubMed] [Google Scholar]
- 78.Basile D, Polano M, Buriolla S, et al. Prognostic role of macrophage infiltration and monocyte-to-lymphocyte ratio in stage III colon cancer: the mirror study [abstract]. J Clin Oncol 2020;38 (Supp 15):P16118. 10.1200/JCO.2020.38.15_suppl.e16118 [DOI] [Google Scholar]
- 79.Wei C, Yang C, Wang S, et al. M2 macrophages confer resistance to 5-fluorouracil in colorectal cancer through the activation of CCL22/PI3K/AKT signaling. Onco Targets Ther 2019;12:3051–63. 10.2147/OTT.S198126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Limagne E, Thibaudin M, Nuttin L, et al. Trifluridine/Tipiracil plus oxaliplatin improves PD-1 blockade in colorectal cancer by inducing immunogenic cell death and depleting macrophages. Cancer Immunol Res 2019;7:1958–69. 10.1158/2326-6066.CIR-19-0228 [DOI] [PubMed] [Google Scholar]
- 81.Correale P, Rotundo MS, Botta C, et al. Tumor infiltration by T lymphocytes expressing chemokine receptor 7 (CCR7) is predictive of favorable outcome in patients with advanced colorectal carcinoma. Clin Cancer Res 2012;18:850–7. 10.1158/1078-0432.CCR-10-3186 [DOI] [PubMed] [Google Scholar]
- 82.Lo Presti E, Pizzolato G, Corsale AM, et al. γδ T Cells and Tumor Microenvironment: From Immunosurveillance to Tumor Evasion. Front Immunol 2018;9:1395. 10.3389/fimmu.2018.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma Y, Aymeric L, Locher C, et al. Contribution of IL-17–producing γδ T cells to the efficacy of anticancer chemotherapy. J Exp Med 2011;208:491–503. 10.1084/jem.20100269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meraviglia S, Lo Presti E, Tosolini M, et al. Distinctive features of tumor-infiltrating γδ T lymphocytes in human colorectal cancer. Oncoimmunology 2017;6:e1347742. 10.1080/2162402X.2017.1347742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang J, Lin C, Li H, et al. Tumor-Infiltrating γδT cells predict prognosis and adjuvant chemotherapeutic benefit in patients with gastric cancer. Oncoimmunology 2017;6:e1353858. 10.1080/2162402X.2017.1353858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edin S, Kaprio T, Hagström J, et al. The Prognostic Importance of CD20+ B lymphocytes in Colorectal Cancer and the Relation to Other Immune Cell subsets. Sci Rep 2019;9:19997. 10.1038/s41598-019-56441-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sautès-Fridman C, Lawand M, Giraldo NA, et al. Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention. Front Immunol 2016;7:407. 10.3389/fimmu.2016.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berntsson J, Eberhard J, Nodin B, et al. Expression of programmed cell death protein 1 (PD-1) and its ligand PD-L1 in colorectal cancer: relationship with sidedness and prognosis. Oncoimmunology 2018;7:e1465165. 10.1080/2162402X.2018.1465165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feng D, Qin B, Pal K, et al. BRAFV600E-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts. Oncogene 2019;38:6752–66. 10.1038/s41388-019-0919-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koganemaru S, Inoshita N, Miura Y, et al. Prognostic value of programmed death-ligand 1 expression in patients with stage III colorectal cancer. Cancer Sci 2017;108:853–8. 10.1111/cas.13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dunne PD, McArt DG, O'Reilly PG, et al. Immune-Derived PD-L1 gene expression defines a subgroup of stage II/III colorectal cancer patients with favorable prognosis who may be Harmed by adjuvant chemotherapy. Cancer Immunol Res 2016;4:582–91. 10.1158/2326-6066.CIR-15-0302 [DOI] [PubMed] [Google Scholar]
- 92.Smyrk TC, Watson P, Kaul K, et al. Tumor-Infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 2001;91:2417–22. [DOI] [PubMed] [Google Scholar]
- 93.Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015;5:43–51. 10.1158/2159-8290.CD-14-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology 1999;117:123–31. 10.1016/S0016-5085(99)70558-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 2015;33:4176–87. 10.1200/JCO.2015.63.4238 [DOI] [PubMed] [Google Scholar]
- 96.Seligmann JF, FOxTROT Collaborative Group . FOxTROT: neoadjuvant FOLFOX chemotherapy with or without panitumumab (Pan) for patients (PTS) with locally advanced colon cancer (CC) [abstract]. J Clin Oncol 2020;38 (Supp 15):P4013. 10.1200/JCO.2020.38.15_suppl.4013 [DOI] [Google Scholar]
- 97.Cercek A, Dos Santos Fernandes G, Roxburgh CS, et al. Mismatch repair-deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin Cancer Res 2020;26:3271–9. 10.1158/1078-0432.CCR-19-3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.des Guetz G, Mariani P, Cucherousset J, et al. Microsatellite instability and sensitivitiy to FOLFOX treatment in metastatic colorectal cancer. Anticancer Res 2007;27:2715–9. [PubMed] [Google Scholar]
- 99.Magrini R, Bhonde MR, Hanski M-L, et al. Cellular effects of CPT-11 on colon carcinoma cells: dependence on p53 and hMLH1 status. Int J Cancer 2002;101:23–31. 10.1002/ijc.10565 [DOI] [PubMed] [Google Scholar]
- 100.Bras-Gonçalves RA, Rosty C, Laurent-Puig P, et al. Sensitivity to CPT-11 of xenografted human colorectal cancers as a function of microsatellite instability and p53 status. Br J Cancer 2000;82:913–23. 10.1054/bjoc.1999.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fallik D, Borrini F, Boige V, et al. Microsatellite instability is a predictive factor of the tumor response to irinotecan in patients with advanced colorectal cancer. Cancer Res 2003;63:5738–44. [PubMed] [Google Scholar]
- 102.Ma J, Zhang Y, Shen H, et al. Association between mismatch repair gene and irinotecan-based chemotherapy in metastatic colon cancer. Tumour Biol 2015;36:9599–609. 10.1007/s13277-015-3723-5 [DOI] [PubMed] [Google Scholar]
- 103.Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: cancer and leukemia group B protocol 89803. J Clin Oncol 2009;27:1814–21. 10.1200/JCO.2008.18.2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klingbiel D, Saridaki Z, Roth AD, et al. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Ann Oncol 2015;26:126–32. 10.1093/annonc/mdu499 [DOI] [PubMed] [Google Scholar]
- 105.Ambe K, Mori M, Enjoji M. S-100 protein-positive dendritic cells in colorectal adenocarcinomas. distribution and relation to the clinical prognosis. Cancer 1989;63:496–503. [DOI] [PubMed] [Google Scholar]
- 106.Nakayama Y, Inoue Y, Minagawa N, et al. Relationships between S-100 protein-positive cells and clinicopathological factors in patients with colorectal cancer. Anticancer Res 2003;23:4423–6. [PubMed] [Google Scholar]
- 107.Gulubova MV, Ananiev JR, Vlaykova TI, et al. Role of dendritic cells in progression and clinical outcome of colon cancer. Int J Colorectal Dis 2012;27:159–69. 10.1007/s00384-011-1334-1 [DOI] [PubMed] [Google Scholar]
- 108.Nagorsen D, Voigt S, Berg E, et al. Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Transl Med 2007;5:62. 10.1186/1479-5876-5-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwaab T, Weiss JE, Schned AR, et al. Dendritic cell infiltration in colon cancer. J Immunother 2001;24:130–7. 10.1097/00002371-200103000-00007 [DOI] [PubMed] [Google Scholar]
- 110.Sandel MH, Dadabayev AR, Menon AG, et al. Prognostic value of tumor-infiltrating dendritic cells in colorectal cancer: role of maturation status and intratumoral localization. Clin Cancer Res 2005;11:2576–82. 10.1158/1078-0432.CCR-04-1448 [DOI] [PubMed] [Google Scholar]
- 111.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007;13:54–61. 10.1038/nm1523 [DOI] [PubMed] [Google Scholar]
- 112.Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 2007;220:47–59. 10.1111/j.1600-065X.2007.00573.x [DOI] [PubMed] [Google Scholar]
- 113.Peng R-Q, Chen Y-B, Ding Y, et al. Expression of calreticulin is associated with infiltration of T-cells in stage IIIB colon cancer. World J Gastroenterol 2010;16:2428–34. 10.3748/wjg.v16.i19.2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer 2009;9:665–74. 10.1038/nrc2714 [DOI] [PubMed] [Google Scholar]
- 115.Park JH, Richards CH, McMillan DC, et al. The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann Oncol 2014;25:644–51. 10.1093/annonc/mdt593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gonçalves-Ribeiro S, Díaz-Maroto NG, Berdiel-Acer M, et al. Carcinoma-Associated fibroblasts affect sensitivity to oxaliplatin and 5FU in colorectal cancer cells. Oncotarget 2016;7:59766–80. 10.18632/oncotarget.11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu Y, Yan C, Mu L, et al. Fibroblast-Derived exosomes contribute to chemoresistance through priming cancer stem cells in colorectal cancer. PLoS One 2015;10:e0125625. 10.1371/journal.pone.0125625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dienstmann R, Villacampa G, Sveen A, et al. Relative contribution of clinicopathological variables, genomic markers, transcriptomic subtyping and microenvironment features for outcome prediction in stage II/III colorectal cancer. Ann Oncol 2019;30:1622–9. 10.1093/annonc/mdz287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsujino T, Seshimo I, Yamamoto H, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res 2007;13:2082–90. 10.1158/1078-0432.CCR-06-2191 [DOI] [PubMed] [Google Scholar]
- 120.Gu J, Li Z, Zhou J, et al. Response prediction to oxaliplatin plus 5-fluorouracil chemotherapy in patients with colorectal cancer using a four-protein immunohistochemical model. Oncol Lett 2019;18:2091–101. 10.3892/ol.2019.10474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xiong Y, Wang K, Zhou H, et al. Profiles of immune infiltration in colorectal cancer and their clinical significant: a gene expression-based study. Cancer Med 2018;7:4496–508. 10.1002/cam4.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Becht E, de Reyniès A, Giraldo NA, et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res 2016;22:4057–66. 10.1158/1078-0432.CCR-15-2879 [DOI] [PubMed] [Google Scholar]
- 123.Allen WL, Dunne PD, McDade S, et al. Transcriptional subtyping and CD8 immunohistochemistry identifies patients with stage II and III colorectal cancer with poor prognosis who benefit from adjuvant chemotherapy. JCO Precis Oncol 2018;2:1–15. 10.1200/PO.17.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Song N, Pogue-Geile KL, Gavin PG, et al. Clinical outcome from oxaliplatin treatment in stage II/III colon cancer according to intrinsic subtypes: secondary analysis of NSABP C-07/NRG oncology randomized clinical trial. JAMA Oncol 2016;2:1162–9. 10.1001/jamaoncol.2016.2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pogue-Geile KL, Andre T, Song N, et al. Association of colon cancer (CC) molecular signatures with prognosis and oxaliplatin prediction-benefit in the mosaic trial (multicenter International study of Oxaliplatin/5FU-LV in the adjuvant treatment of colon cancer) [abstract]. J Clin Oncol 2019;37 (Supp 15):P3503. 10.1200/JCO.2019.37.15_suppl.3503 [DOI] [Google Scholar]
- 126.Stintzing S, Wirapati P, Lenz H-J, et al. Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann Oncol 2019;30:1796–803. 10.1093/annonc/mdz387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lenz H-J, Ou F-S, Venook AP, et al. Impact of consensus molecular subtype on survival in patients with metastatic colorectal cancer: results from CALGB/SWOG 80405 (Alliance). J Clin Oncol 2019;37:1876–85. 10.1200/JCO.18.02258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marisa L, Ayadi M, Balogoun R, et al. Clinical utility of colon cancer molecular subtypes: validation of two main colorectal molecular classifications on the PETACC-8 phase III trial cohort [abstract]. J Clin Oncol 2017;35 (Supp 15):P3509. 10.1200/JCO.2017.35.15_suppl.3509 [DOI] [Google Scholar]
- 129.Trinh A, Trumpi K, De Sousa E Melo F, et al. Practical and robust identification of molecular subtypes in colorectal cancer by immunohistochemistry. Clin Cancer Res 2017;23:387–98. 10.1158/1078-0432.CCR-16-0680 [DOI] [PubMed] [Google Scholar]
- 130.Okita A, Takahashi S, Ouchi K, et al. Consensus molecular subtypes classification of colorectal cancer as a predictive factor for chemotherapeutic efficacy against metastatic colorectal cancer. Oncotarget 2018;9:18698–711. 10.18632/oncotarget.24617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Del Rio M, Mollevi C, Bibeau F, et al. Molecular subtypes of metastatic colorectal cancer are associated with patient response to irinotecan-based therapies. Eur J Cancer 2017;76:68–75. 10.1016/j.ejca.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 132.Hof J, Kok K, Sijmons RH, et al. Systematic review of the prognostic role of the immune system after surgery of colorectal liver metastases. Front Oncol 2019;9:148. 10.3389/fonc.2019.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mlecnik B, Van den Eynde M, Bindea G. Comprehensive Intrametastatic immune quantification and major impact of immunoscore on survival. J Natl Cancer Inst 2018;110:97–108 10.1093/jnci/djx123. [DOI] [PubMed] [Google Scholar]
- 134.Halama N, Michel S, Kloor M, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res 2011;71:5670–7. 10.1158/0008-5472.CAN-11-0268 [DOI] [PubMed] [Google Scholar]
- 135.Shahda S, Noonan AM, Bekaii-Saab TS, et al. A phase II study of pembrolizumab in combination with mFOLFOX6 for patients with advanced colorectal cancer [abstract]. J Clin Oncol 2017;35:P3541. 10.1200/JCO.2017.35.15_suppl.3541 [DOI] [Google Scholar]
- 136.Pfirschke C, Engblom C, Rickelt S, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 2016;44:343–54. 10.1016/j.immuni.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]