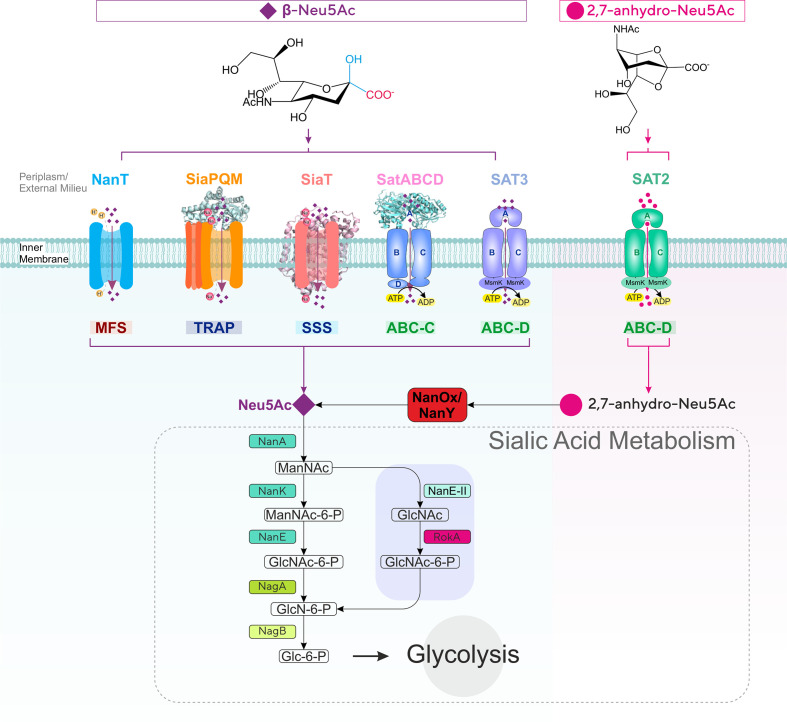
Fig. 1.
Diversity of sialic acid transport and catabolism in bacteria. Sialic acid-utilizing bacteria have evolved multiple types of transporters functioning at the inner (cytoplasmic) membrane from four major (super)families (MFS, TRAP, ABC and SSS) differing by mode of energization and subunit composition. Once inside the cell, sialic acid is metabolized to GlcNAc-6-P via one of two characterized pathways to then enter central metabolism by the action of the GlcNAc-catabolic enzymes NagA (GlcNAc-6-P deacetylase) and NagB (GlcN-6-P deaminase). In the E. coli paradigm [27], Neu5Ac is converted by the sequential action of three dedicated enzymes, namely NanA (Neu5Ac lyase), NanK (ManNAc kinase) and NanE (ManNAc-6-P epimerase); in the Bacteroidetes paradigm [30, 31], NanA and the alternative enzyme NanE-II (GlcNAc epimerase) feed substrate to the glucokinase RokA. Bacteria such as Ruminococcus gnavus that take up 2,7-anhydro-Neu5Ac use the cytoplasmic oxidoreductase NanOx (NanY in E. coli) to convert the substrate to Neu5Ac, which then enters a canonical Nan pathway. ManNAc, N-acetyl-mannosamine; GlcNAc, N-acetyl-glucosamine; GlcN, glucosamine; Glc, glucose; MsmK, multitasking ATPase protein serving multiple sugar ABC transporters including sialic acid transporters [44].