Abstract
Bovine milk exosomes (BMEs) are being explored in drug delivery despite their rapid elimination by macrophages. We aimed at identifying the BME transporter in murine bone marrow-derived macrophages (BMDMs). Fluorophore-labeled BMEs were used in transport studies in BMDMs from C57BL/6J and class A scavenger receptor type 1/2 (CASR-1/2) knockout mice and tissue accumulation in macrophage-depleted C57BL/6J mice. Parametric and nonparametric statistics tests for pairwise and multiple comparisons were used. Chemical inhibitors of phagocytosis by cytochalasin D led to a 69 ± 18% decrease in BME uptake compared with controls (P < 0.05), whereas inhibitors of endocytic pathways other than phagocytosis had a modest effect on uptake (P > 0.05). Inhibitors of class A scavenger receptors (CASRs) including CASR-1/2 caused a 70% decrease in BME uptake (P < 0.05). The uptake of BMEs by BMDMs from CASR-1/2 knockout mice was smaller by 58 ± 23% compared with wild-type controls (P < 0.05). Macrophage depletion by clodronate caused a more than 44% decrease in BME uptake in the spleen and lungs (P < 0.05), whereas the decrease observed in liver was not statistically significant. In conclusion, CASR-1/2 facilitates the uptake of BMEs in BMDMs and C57BL/6J mice.
Keywords: bone marrow-derived macrophages, bovine, class A scavenger receptors, phagocytosis, milk exosomes
INTRODUCTION
Exosomes and exosome-mimetic nanoplatforms have garnered attention as vehicles for delivering therapeutics to malignant tumors including the transfer of therapeutics across the blood-brain barrier (BBB) (1). For example, transgenic exosomes from self-derived dendritic cells delivered short interfering RNA (siRNA) to neurons, microglia, oligodendrocytes, and their precursors following intravenous injection and knocked down target gene expression by ∼60% (2). The utility of the technology in cancer treatment was illustrated by the demonstration that clinical-grade exosomes, loaded with siRNA specific to oncogenic KRASG12D, slowed tumor growth by more than 60% compared with controls receiving scrambled RNA and doubled survival times from 250 days to 500 days after tumor induction in multiple mouse models of pancreatic cancer (3).
Bovine milk exosomes (BMEs) have sparked commercial and scientific interest as promising candidates for delivering siRNA therapeutics to tumors (4, 5). BMEs offer distinct advantages in drug delivery compared with synthetic nanovesicles and exosomes purified from mammalian cell cultures. BMEs and their RNA cargos may be bioavailable after oral administration (6–8), although bioavailability is still somewhat controversial (discussed in Ref. 9). The production of BMEs is scalable and cost-effective considering that 1 mL of milk yields 1012 BME-sized extracellular vesicles and a cow produces ∼6,000 kg of milk per season (10, 11). BMEs did not elicit immune responses in human mononuclear cells ex vivo and in mice (12). Paclitaxel-loaded BMEs caused a 60% decrease in A549 lung cancer xenografts in athymic nude mice compared with free paclitaxel 7 wk after initiation of oral BME treatment (5).
Exogenous exosomes are recognized as foreign particles and eliminated by macrophages (13, 14). To date, studies of BME uptake by macrophages have been limited to the assessment of BME elimination in the human macrophage-like differentiated THP-1 cell line (15). Although that study suggests that macrophages take up BMEs, it does not offer insights into transport mechanisms and kinetics and the elimination of BMEs by macrophages in a whole organism. The hepatic and splenic accumulation of intravenously and orally administered BMEs suggests that uptake by resident liver and spleen macrophages, Kupffer cells and several subsets of splenic macrophages, contributes considerably to the elimination of BMEs from circulation (7). The clearance of BMEs by Kupffer cells and other tissue-resident macrophages decrease the efficacy and specificity of drug-loaded BMEs meant to accumulate in tumors. For example, the clearance of B16BL6 murine melanoma cell-derived exosomes from blood circulation decreased 63-fold when Balb/c mice were depleted of macrophages by using clodronate compared with controls (14).
Here, we assessed transport mechanisms and kinetics of BME uptake in murine bone marrow-derived macrophages and the biodistribution of BME in macrophage-depleted mice. The goal of the project is to generate insights into possible approaches to maximizing the efficacy of drug delivery through BMEs.
MATERIALS AND METHODS
Animal Husbandry
C57BL/6J (Stock No. 000664) and class A scavenger receptor type 1/2 (CASR-1/2) knockout (KO) mice (Stock No. 006096) (16) were purchased from the Jackson Laboratory and housed in a specific pathogen-free facility at 22°C in a 12-h light/dark cycle. The mice had free access to Teklad Global 16% Protein Rodent Diet (Cat. No. Teklad 2016, Envigo) and water. Both male and female mice ages 8–20 wk were used to collect bone marrow cells for in vitro studies. Three male and three female mice ages 8–20 wk were used for the in vivo distribution studies. Animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska-Lincoln (Protocol Nos. 1713 and 1421).
Preparation of BMDMs
BMDMs were prepared as previously described (17). Briefly, the femur and tibia of C57BL/6J and CASR-1/2 KO mice were crushed to release bone marrow cells, which were cultured using RPMI supplemented with 10% fetal bovine serum (FBS), 50 µg/mL penicillin-streptomycin, 2 nM glutamine, 25% L929 cell-conditioned medium, and 1 mM sodium pyruvate. Cells were cultured at 37°C in 5% CO2 for 6–8 days until BMDMs reached 80%–90% confluence. BMDMs were harvested, reseeded in culture plates, and used for BMEs transport studies on the following day. The purity of differentiated BMDMs was assessed by using flow cytometry and staining with anti-F4/80 antibody (BioLegend, Inc.); controls were prepared in the absence of antibody or by staining with IgG2a isotype antibody (BioLegend, Inc.). Data were analyzed by using FlowJo software (FlowJo, LLC) and ∼94% of events tested positive for F4/80 (Supplemental Figs. S1 and S7; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.14763363.v5). The Thiazolyl Blue Tetrazolium Bromide (MTT) assay (Sigma-Aldrich) was used to assess the viability and metabolic activity of BMDMs as previously described (18).
Isolation and Labeling of BMEs
Skim bovine milk was obtained from a local grocery store and BMEs were isolated by differential ultracentrifugation as previously described (8). BMEs were authenticated transmission electron microscopy, nanoparticle tracking analysis (Nanosight NS300, Malvern Instruments Ltd), and immunoblot analysis as previously described (19) (Supplemental Fig. S2); protocols have been deposited in EV-Track (EVTrack ID: EV210157; Ref. 20). BME preparations tested positive for three exosome markers (CD63, CD9, and Tsg101) and αs1-casein. The latter is consistent with a previous report that milk exosomes contain casein (21) but one cannot exclude with absolute certainty that exosome preparations were contaminated with casein micelles. Markers for microvesicles (integrin β1) and nuclear material (histone H3) were not detected by immunoblot analysis. Exosome pellets were suspended in sterile phosphate-buffered saline (PBS) and stored at −80°C up to 6 mo before use.
Glycoproteins on the BME surface were labeled with carbonyl-reactive HiLyte Fluor 750 hydrazide (AnaSpec, Inc.; Cat. No. AS-81268) at a final concentration of 0.16 mM at 37°C for 30 min. RNA cargos were labeled by incubating BMEs with Exo-Red (System Biosciences; Cat. No. EXOR100A-1) at 37°C for 10 min. Note that the Exo-Red dye binds to every species of single-stranded RNA and, therefore, does not discriminate among distinct RNAs; the reagent also transfers from RNA to DNA after a certain incubation time. Exo-Red (EXOR100A-1) has been discontinued and replaced with another product that exclusively labels mRNA cargos (Cat. No. EXOGR800A-1). BME membrane lipids were labeled with PKH26 following the manufacturer’s protocol (Sigma-Aldrich). Excess HiLyte, Exo-Red, and PKH26 were removed by washing BMEs with PBS and centrifugation (100,000 g for 90 min). BMEs were resuspended in PBS, sterile-filtered by using a 0.2-µm membrane filter (VWR), and stored at −80°C until use. The concentration of BME protein was measured by using the Qubit Protein Assay Kit (Cat. No. Q33212; Thermo Fisher Scientific). The concentration and size of BMEs were analyzed by using nanoparticle tracking analysis.
BME Transport Studies
Studies of HiLyte-labeled BME and Exo-Red-labeled BME uptake were conducted using 2.1 × 109 to 4.3 × 1011 BMEs/mL and 0.2 × 105 BMDMs at 37°C for 2 to 53 h as described in results. Briefly, 0.2 × 105 and 1 × 105 BMDMs were seeded in 96- and 24-well plates, respectively. Cells were allowed to adhere overnight when fluorophore-labeled BMEs and transport inhibitors were added and transport was measured using the conditions shown in results. Unlabeled BMEs were used to assess background noise. BMDMs were washed twice with PBS and fluorescence intensities were measured. The uptake of HiLyte-labeled BMEs was measured using the 800-nm channel in a LI-COR Odyssey CLx system (LI-COR Biosciences), and the uptake of Exo-Red-labeled BMEs was measured in a Biotek FLx800 reader (Bio-Tek Instruments; excitation 450 nm, emission 645 nm). Fluorescence readings were corrected by subtracting the background noise produced by cells incubated with unlabeled BMEs. Fluorescence was converted to BMEs by using a known number of fluorophore-labeled BMEs in PBS. In temporal studies, the number of BMDMs present at the time of measurement was estimated by extracting cell proteins with RIPA buffer containing a protease inhibitor cocktail (Cat. No. P8340-1ML; Sigma-Aldrich) and the bicinchoninic acid method (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific); assays were calibrated by using a known number of BMDMs. Transport rates in time-course and dose-response experiments are shown in units of BMEs × (20,000 BMDMs)−1 and BMEs × (20,000 BMDMs × 2 h)−1, respectively.
For confocal microscopy, BMDMs were seeded on poly-lysine coated cover glasses and fixed with 4% p-formaldehyde solution for 20 min. Prolong gold antifade DAPI mount reagent (Thermo Fisher Scientific) was used to mount and stain nuclei. Digital images were acquired at a frame size of 1,024 × 1,024 pixels (0.21 µm/pixel) using a z-stack protocol and a Nikon ECLIPSE Ti2 microscope (NIKON) in the Morrison Microscopy Core Research Facility of the University of Nebraska-Lincoln. The confocal images were analyzed using ImageJ (22).
Cytochalasin D (Sigma-Aldrich), 5-ethyl-N-isopropyl amiloride (EIPA; Santa Cruz), chlorpromazine (Sigma-Aldrich), and nystatin (Sigma-Aldrich) were used to inhibit phagocytosis (23), micropinocytosis (24), clathrin-mediated endocytosis (25), and caveolar and lipid raft-dependent endocytosis (26), respectively. Inhibitor concentrations are shown in results. Stock solutions were prepared by dissolving cytochalasin D, nystatin, and EIPA in dimethyl sulfoxide, whereas chlorpromazine was dissolved in culture media. BMDMs were incubated with inhibitors or solvent at 37°C for 30 min when HiLyte-BMEs were added (2 × 1011/mL, final concentration) and incubation was continued for 4 h. High doses of some inhibitors led to a decrease in the metabolic activity of BMDMs; any treatment that led to a decrease greater than 30%, judged by MTT assay (Sigma-Aldrich), was excluded from the analysis of BME uptake (see results) (27). Our data suggest that phagocytosis plays a crucial role in the uptake of BMEs by BMDMs (see results), and we further assessed the role of phagocytosis by using two blockers of CASRs including CASR-1/2, fucoidan (Sigma-Aldrich) and dextran sulfate (Sigma) (28). Fucoidan and dextran sulfate were dissolved in culture media.
In Vivo Distribution of HiLyte-BMEs in Macrophage-Depleted Mice
Clodronate-loaded liposomes were prepared as previously described (29). Mice were depleted of macrophages by a single intraperitoneal administration of 0.15 mL clodronate-loaded liposomes per 10 g body wt; controls were injected with PBS (29, 30). Macrophages in splenic single-cell suspensions were stained using anti-F4/80 antibody (Biolegend Cns, Inc.); controls were prepared by not staining the cells or by staining with IgG2a isotype antibody (Biolegend Cns, Inc.). The number of F4/80-positive cells was quantified by using FlowJo software (FlowJo, LLC). HiLyte-labeled BMEs (4 × 1011 BMEs/g body wt) were administered by oral gavage 48 h after clodronate treatment; controls received unlabeled BMEs. Mice were euthanized 12 h after BME administration and tissues were dissected, rinsed with PBS, and analyzed in a LI-COR Odyssey CLx system (LI-COR Biosciences) as previously described (7). Blood was collected by cardiac puncture and plasma was separated by centrifugation at 600 g for 15 min. Fluorescence readings were corrected for background signal by subtracting signals produced by unlabeled BME in mice matched for age and body weight. The choice of BME dose and the collection time was informed by our previous dose-response, time-course studies (7).
Statistical Analyses
BME uptake was linear for dose and time under the conditions tested here; curves were fitted using linear regression analysis. The normality of data distribution was tested by using the Shapiro–Wilk test (31). Homogeneity of variances of data was assessed and confirmed by using the Brown–Forsythe test (32). Parametric data were analyzed using unpaired t test for comparison of two groups and one-way ANOVA with Dunnett’s multiple comparisons post hoc test for comparisons among more than two groups. Nonparametric data were analyzed using two-tailed Mann–Whitney U test for comparison of two groups and Kruskal–Wallis test with Dunn’s multiple comparisons post hoc test for comparisons among more than two groups. GraphPad Prism 6 was used for all statistical analysis (GraphPad Software, Inc.). Differences were considered significant if P ≤ 0.05. Data are presented as means ± SD.
RESULTS
BME Uptake by BMDMs
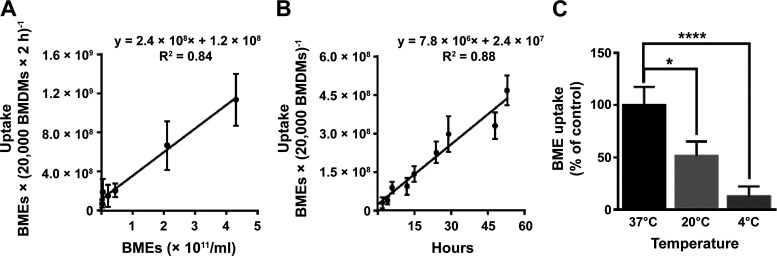
The uptake of HiLyte-labeled BMEs by BMDMs was not saturated under physiological conditions (Fig. 1, A and B). We assessed BME uptake at concentrations comparable with exosome concentrations in human plasma and times up to nine half-lives of exogenous exosomes in mice (14, 33, 34). Note that supraphysiological concentrations of BMEs led to a decrease in the metabolic activity of BMDMs and, therefore, were not used in transport studies (Supplemental Fig. S3). Although BME uptake was not saturated under the experimental conditions used here, transport rates depended on the incubation temperature (Fig. 1C), which is consistent with carrier-facilitated uptake. Previous studies showed that BME uptake by intestinal cells depends on glycoproteins on the exosome surface (8), suggesting that the carbonyl-reactive HiLyte label might impact BME uptake. Consistent with this theory, we observed a 50% lower uptake rate for HiLyte-labeled BMEs compared with Exo-Red labeled BMEs (Supplemental Fig. S4). The rate of uptake was 1.1 × 109 ± 2.7 × 108 and 2.5 × 109 ± 4.3 × 108 BMEs (20,000 BMDMs × 2 h)−1 for BMEs labeled with HiLyte and Exo-Red, respectively, at a substrate concentration of 4.3 × 1011 BMEs/mL (Fig. 1A and Supplemental Fig. S4).
Figure 1.
Transport kinetics of BME uptake in murine BMDMs. BMEs were covalently labeled using HiLyte Fluor 750 hydrazide and added in BMDM culture. Values are means ± SD. A: uptake of BMEs as a function of substrate concentration at 37°C for 2 h. B: uptake of BMEs as a function of time at a concentration of 1.7 × 1010 BMEs/mL at 37°C. C: uptake of BMEs as a function of incubation temperature. BMDM cultures were kept at 4°C, 22°C, and 37°C for 30 min before the addition of HiLyte-labeled BMEs (3.5 × 1011 BMEs/mL) and incubation for 2 h; data are expressed as a percent of uptake measured at 37°C. All data represent 3 independent experiments, each performed in triplicate. Transport kinetics in A and B were analyzed by linear regression and comparison among groups in C was performed by the Kruskal–Wallis test with Dunn’s multiple comparisons post hoc test. *P < 0.05, ****P < 0.0001. BMDM, bone marrow-derived macrophage; BME, bovine milk exosome.
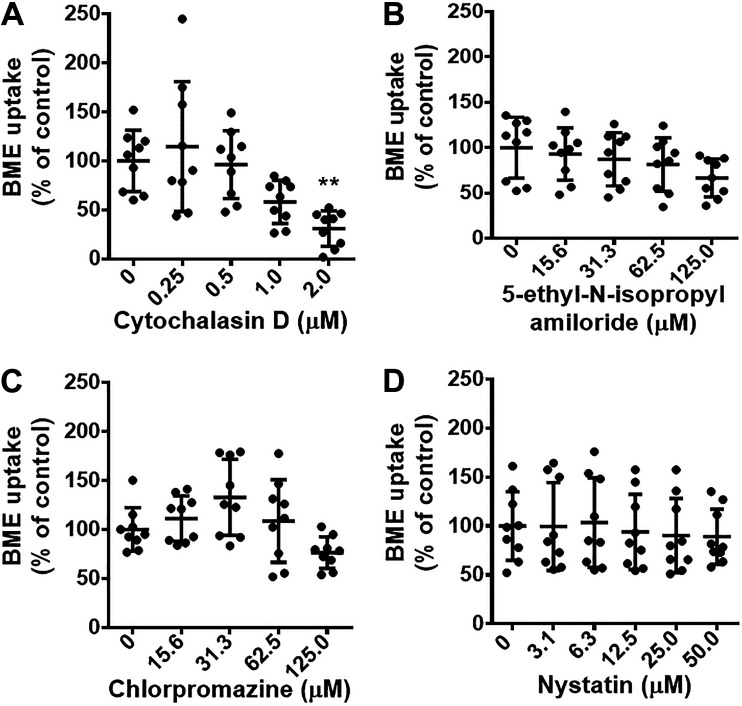
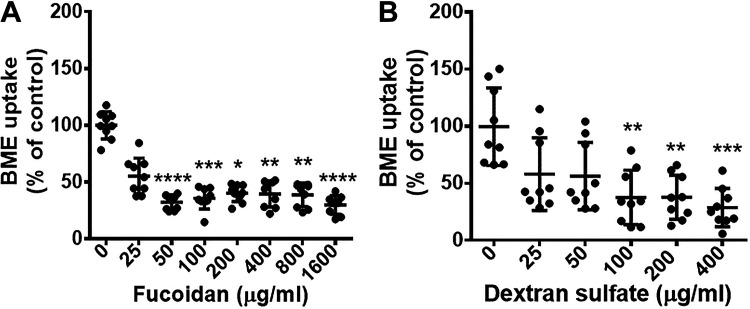
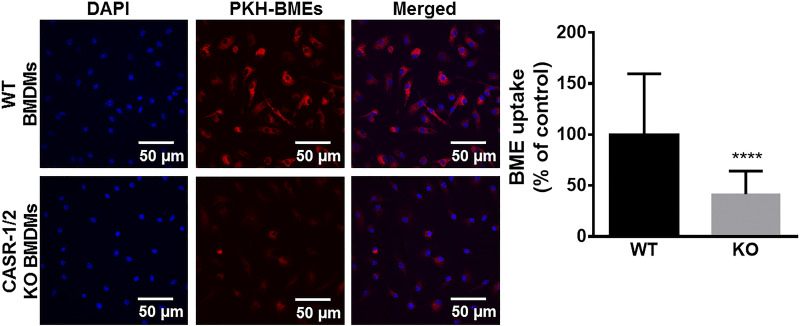
The uptake of BMEs by BMDMs depends on endocytosis, particularly phagocytosis. We considered BME uptake by endocytosis a prime candidate based on the rationale that endocytosis is an important pathway of exosome uptake by recipient cells and endocytosis activity is high in macrophages (35, 36). Inhibitors of phagocytosis caused a significant decrease in BME uptake (Fig. 2A), whereas inhibitors of endocytosis by pathways other than phagocytosis had no significant effect on BME uptake (Fig. 2, B–D). We cannot formally exclude the possibility that pathways other than phagocytosis contribute to BME uptake by BMDMs. Such studies would require the use of inhibitors at concentrations that had adverse effects on BMDM metabolic activity (Fig. 2, B–D, and Supplemental Fig. S5). Rather, we continued toward identifying the transporter that facilitates BME phagocytosis. We hypothesized that CASR-1/2 facilitate BME uptake because that class of receptors is known to play a role in the transport of negatively charged macromolecules such as exosomes (37, 38). Treatment of BMDMs with inhibitors of CASRs including CASR-1/2, fucoidan, and dextran sulfate caused a significant decrease in the uptake of HiLyte-labeled BMEs compared with solvent controls (Fig. 3, A and B) without compromising the metabolic activity in BMDMs (Supplemental Fig. S6). We confirmed the role of CASR-1/2 in BME phagocytosis by using a genetics approach. The uptake of PKH26-labeled BMEs was significantly lower in BMDMs derived from CASR-1/2 KO mice compared with BMDMs derived from wild-type C57BL/6J mice (Fig. 4).
Figure 2.
Pathways of BME endocytosis in murine BMDMs. BMDMs were pretreated 30 min with inhibitors of phagocytosis (cytochalasin D; A), macropinocytosis (5-ethyl-N-isopropyl amiloride; B), clathrin-dependent endocytosis (chlorpromazine; C), and caveolin and lipid raft-dependent endocytosis (nystatin; D) before the addition of HiLyte-labeled BMEs (2 × 1011/mL) and incubation at 37°C for 4 h. Data in panels represent 3 independent experiments, each performed in triplicate. Results in inhibitor-treated cells are expressed as percent of solvent controls. Values are means ± SD. Data were analyzed by one-way ANOVA and post hoc testing by Dunnett’s test (A and B) or Kruskal–Wallis test with Dunn’s multiple comparisons test (C and D). **P < 0.01. BMDM, bone marrow-derived macrophage; BME, bovine milk exosome.
Figure 3.
Effects of class A scavenger receptor (CASR) inhibitors on the internalization of BMEs in murine BMDMs. BMDMs were pretreated for 30 min with inhibitors of class A scavenger receptors (A, fucoidan; B, dextran sulfate) before the addition of HiLyte-labeled BMEs (2 × 1011/mL) and incubation at 37°C for 4 h. Data in panels represent 3 independent experiments, each performed in triplicate. Results in inhibitor-treated cells are expressed as percent of solvent controls. Values are means ± SD. Data were analyzed by Kruskal–Wallis test and post hoc testing by Dunn’s test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. BMDM, bone marrow-derived macrophage; BME, bovine milk exosome.
Figure 4.
Effect of class A scavenger receptor 1/2 knockout on BME uptake in murine BMDMs. BMDMs were differentiated from bone marrow cells collected from C57BL/6J wild-type (WT) or class A scavenger receptor type-1/2 knockout (CASR-1/2 KO) mice. BMDMs were incubated with PKH-26-labeled BME (red) (2 × 1011/mL) for 4 h at 37°C; controls were incubated with unlabeled BMEs. Left: confocal microscopy images of PKH-26-labeled BME uptake in WT and CASR-1/2 KO BMDMs. Nuclei (blue) were stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar: 50 µm. Right: the PKH signal from >100 BMDMs was quantified using ImageJ. Results in CASR-1/2 KO cells are expressed as percent of WT controls. Values are means ± SD. Data were analyzed by Mann–Whitney U test. ****P < 0.0001. BMDM, bone marrow-derived macrophage; BME, bovine milk exosome.
BME Uptake by Tissue Macrophages in Vivo
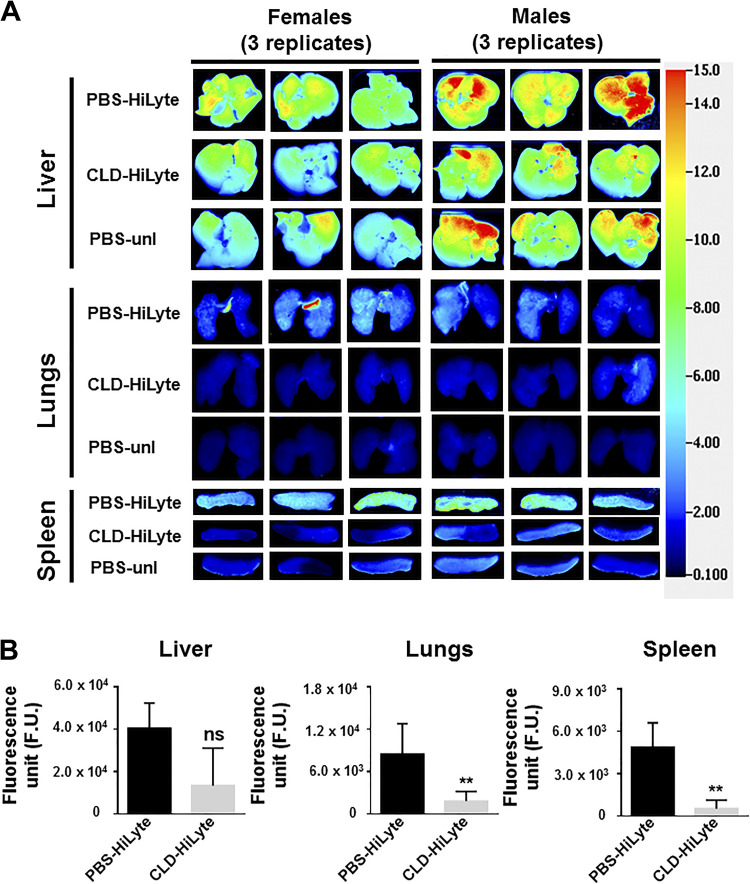
Clodronate treatment resulted in a decrease in the accumulation of HiLyte-labeled BMEs in macrophage-rich tissues (Fig. 5, A and B). Clodronate treatment caused an 84 ± 15% and 94 ± 10% decrease of BME accumulation in the spleen in male and female mice, respectively, compared with controls. Likewise, clodronate treatment caused a 68 ± 24% and 88 ± 1.9% decrease of BME accumulation in the lungs in male and female mice, respectively, compared with controls. Clodronate treatment also caused a decrease of BME accumulation in other tissues such as kidneys and pancreas (not shown). Although clodronate treatment caused an 89 ± 19% and 47 ± 50% decrease of BME accumulation in the liver in male and female mice, respectively, compared with controls. The hepatic decrease was not statistically significant. Treatment of mice with clodronate-loaded liposomes led to the elimination of splenic macrophages. The number of F4/80-positive cells decreased by 77 ± 21% in spleens from clodronate-treated mice compared with mice treated with PBS (Supplemental Fig. S8).
Figure 5.
Tissue distribution of BMEs in macrophage-depleted mice. Adult male and female C57BL/6J mice were treated with clodronate-loaded liposomes in PBS to achieve macrophage depletion; controls were treated with PBS. Forty-eight hours after clodronate treatment, HiLyte-labeled BMEs or unlabeled BMEs (4 × 1011 BMEs/g) were administered by oral gavage. Mice were euthanized 12 h after oral gavage and the distribution of HiLyte-labeled BMEs was analyzed in excised tissues. A: representative images of liver, lungs, and spleen of female and male mice are shown (3 mice per sex). Rows for each tissue represent the following treatments: row 1 = mice not treated with clodronate (PBS controls) and receiving HiLyte-labeled BMEs (PBS-HiLyte); and row 2 = mice treated with clodronate-liposome and receiving HiLyte-labeled BMEs (CLD-HiLyte); row 3 = mice not treated with clodronate (PBS controls) and receiving unlabeled BMEs (PBS-unl). B: densitometry analysis of background-corrected fluorescence units (F.U.) in liver, lungs, and spleen of male and female mice (combined). Values are means ± SD. Data were analyzed by Mann–Whitney U test. **P < 0.01. BME, bovine milk exosome; ns, nonsignificant.
DISCUSSION
This report is first to identify CASR-1/2 as the receptors responsible for the uptake and elimination of BMEs by host macrophages. This is an important discovery because BMEs are being explored for use in the delivery of drugs to tumors (5, 39, 40). The power of this delivery strategy is reduced by the rapid clearance of exosomes by macrophages (14) and the high capacity of exosome transporters in macrophages as shown in this report. Now that we demonstrated the participation of CASR-1/2 in BME uptake by macrophages, it may be possible to develop strategies for decreasing the rate of BME elimination in patients.
Strengths of this study include the combination of BME labeling protocols, transport inhibitors, and receptor KO cells to identify the BME transporter in BMDM cultures in conjunction with macrophage depletion studies in whole animals. We minimized the use of lipophilic exosome labels because these labels may transfer from exosome membranes to other lipophilic compounds such lipoproteins and some proteins (41). Rather, we obtained an accurate estimate of BME transport rates by comparing uptake rates of BMEs labeled with HiLyte and Exo-Red in BMDMs. The HiLyte label attaches covalently to carbonyl groups on the outer surface of exosomes which comes at the expense of altering the BME surface chemistry. The Exo-Red label offers the advantage of attaching to RNAs inside exosomes but has the limitation that Exo-Red transfers from RNA to DNA on uptake by cells. Dye transfer to DNA is complete ∼24 h after internalization of exosomes by cells as per the vendor’s user manual. As expected, apparent transport rates of HiLyte-labeled BMEs were smaller than the rates measured for BMEs labeled with Exo-Red. That said, the dose-response patterns of BME uptake were the same for both labels. One might speculate that the manipulation of glycans on the BME surface, such as by HiLyte, might be a feasible strategy for decreasing the rate of BME uptake in patients with cancer. This theory is consistent with a previous report suggesting that glycans on the BME surface play a role in the uptake of BMEs by human and rodent intestinal cells (8). The analysis of BME glycoproteins and their roles in BME transport is an ongoing line of investigation in our laboratory. We did not conduct time-course studies with Exo-Red labeled BMEs, because of the transfer of the dye from RNA to DNA.
Our results are consistent with a previous report which suggested that ovarian cancer cells take up exosomes by endocytosis (35). The previous report stressed the importance of clathrin-dependent endocytosis and acknowledged that other endocytic pathways also play a role in exosome transport. The previous report relied on chemical inhibitors and colocalization studies for interrogating pathways of endocytosis. In our studies, we also tested chemical inhibitors for initial screening before using KO mice to implicate CASR-1/2 in the transport of BMEs. We acknowledge that endocytic pathways other than CASR-1/2-dependent phagocytosis may also play a role in the uptake of BMEs by macrophages. This theory is consistent with the modest decrease of BME uptake by BMDMs treated with inhibitors of endocytic pathways other than phagocytosis in this study.
So, what are the limitations and future directions of this line of research? First, we would have expected that the depletion of macrophages by clodronate treatment would not only result in a decreased accumulation of BMEs in resident macrophages in spleen, lungs, and liver but also in an increased accumulation in other tissues. The opposite was the case and we cannot explain why clodronate treatment caused a decrease of BME accumulation in tissues that typically do not harbor large numbers of macrophages. Second, we cannot explain the observed sex-specific difference in BME tissue distribution in vivo. Third, we have yet to assess the fate of BMEs on internalization by macrophages. Possible scenarios include extensive degradation in lysosomes, release of cargos into the cytoplasm, and secretion into the extracellular space. Third, one may want to leverage the accumulation of BMEs in macrophages to deliver drugs to this cell lineage.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S8: https://doi.org/10.6084/m9.figshare.14763363.v5.
GRANTS
This work was supported by NIH 2P20GM104320, National Institute of Food and Agriculture 2016-67001-25301 and 2020-67017-30834, United States Department of Agriculture (USDA) Hatch-1011996, and USDA W4002 (to J.Z.), USDA Hatch-1015948 and USDA Multistate Hatch-1021080 (to J.Y.).
DISCLOSURES
J.Z. serves as a consultant for PureTech Health, Inc. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.Z. conceived and designed research; A.K. performed experiments; A.K. and J.Z. analyzed data; A.K., J.Y., and J.Z. interpreted results of experiments; A.K. prepared figures; A.K. drafted manuscript; J.Z. edited and revised manuscript; A.K., J.Y., and J.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Xingyi Chen and Baolong Liu for supplying L929 conditioned media, and Sonal Sukreet for her suggestions regarding experimental design. We also acknowledge Terri Fangman, Dirk Anderson, and You Zhou for their assistance with confocal microscopy, flow-cytometry, and transmission electron microscopy, respectively. We thank the Biomedical and Obesity Research Core (NIH P20GM104320) for their service.
REFERENCES
- 1.Vázquez-Ríos AJ, Molina-Crespo Á, Bouzo BL, López-López R, Moreno-Bueno G, de la Fuente M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J Nanobiotechnology 17: 85, 2019. doi: 10.1186/s12951-019-0517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29: 341–345, 2011. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 3.Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS, Kalluri R. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 3: e99263, 2018. doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ThePharmaletter. PureTech out-licenses milk exosomes technology in $1 billion deal (Online), 2018. https://www.thepharmaletter.com/article/puretech-out-licenses-milk-exosomes-technology-in-1-billion-deal. [2021 Aug 24].
- 5.Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, Alhakeem SS, Oben K, Munagala R, Bondada S, Gupta RC. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine 13: 1627–1636, 2017. doi: 10.1016/j.nano.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Baier SR, Nguyen C, Xie F, Wood JR, Zempleni J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr 144: 1495–1500, 2014. doi: 10.3945/jn.114.196436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manca S, Upadhyaya B, Mutai E, Desaulniers AT, Cederberg RA, White BR, Zempleni J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep 8: 11321, 2018. doi: 10.1038/s41598-018-29780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf T, Baier SR, Zempleni J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma Caco-2 cells and rat small intestinal IEC-6 cells. J Nutr 145: 2201–2206, 2015. doi: 10.3945/jn.115.218586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zempleni J, Sukreet S, Zhou F, Wu D, Mutai E. Milk-derived exosomes and metabolic regulation. Annu Rev Anim Biosci 7: 245–262, 2019. doi: 10.1146/annurev-animal-020518-115300. [DOI] [PubMed] [Google Scholar]
- 10.Vaswani K, Mitchell MD, Holland OJ, Qin Koh Y, Hill RJ, Harb T, Davies PSW, Peiris H. A method for the isolation of exosomes from human and bovine milk. J Nutr Metab 2019: 5764740, 2019. doi: 10.1155/2019/5764740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh YQ, Peiris HN, Vaswani K, Meier S, Burke CR, Macdonald KA, Roche JR, Almughlliq F, Arachchige BJ, Reed S, Mitchell MD. Characterization of exosomes from body fluids of dairy cows. J Anim Sci 95: 3893–3904, 2017. doi: 10.2527/jas2017.1727. [DOI] [PubMed] [Google Scholar]
- 12.Mutai E, Ramer-Tait AE, Zempleni J. MicroRNAs in bovine milk exosomes are bioavailable in humans but do not elicit a robust pro-inflammatory cytokine response. BMC exRNA 2: 2, 2020. doi: 10.1186/s41544-019-0041-x. [DOI] [Google Scholar]
- 13.Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CIE, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4: 26316, 2015. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T, Matsumoto A, Charoenviriyakul C, Takakura Y. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles 4: 26238, 2015. doi: 10.3402/jev.v4.26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumi H, Tsuda M, Sato Y, Kosaka N, Ochiya T, Iwamoto H, Namba K, Takeda Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J Dairy Sci 98: 2920–2933, 2015. doi: 10.3168/jds.2014-9076. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Horiuchi S, Takahashi K, Kruijt JK, van Berkel TJC, Steinbrecher UP, Ishibashi S, Maeda N, Gordon S, Kodama T. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386: 292–296, 1997. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 17.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med 185: 1977–1985, 1997. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tada H, Shiho O, Kuroshima K, Koyama M, Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods 93: 157–165, 1986. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- 19.Leiferman A, Shu J, Upadhyaya B, Cui J, Zempleni J. Storage of extracellular vesicles in human milk, and microRNA profiles in human milk exosomes and infant formulas. J Pediatr Gastroenterol Nutr 69: 235–238, 2019. doi: 10.1097/MPG.0000000000002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EV-TRACK Consortium; Van Deun J, Mestdagh P, Agostinis P, Akay Ö, Anand S, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods 14: 228–232, 2017.doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 21.Reinhardt TA, Lippolis JD, Nonnecke BJ, Sacco RE. Bovine milk exosome proteome. J Proteomics 75: 1486–1492, 2012. doi: 10.1016/j.jprot.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Rasband W. ImageJ 1.39t software. Bethesda, MD: National Institutes of Health, 2008. http://rsb.info.nih.gov/ij/. [Google Scholar]
- 23.Schliwa M. Action of cytochalasin D on cytoskeletal networks. J Cell Biol 92: 79–91, 1982. doi: 10.1083/jcb.92.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic 10: 364–371, 2009. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol 123: 1107–1117, 1993. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta 864: 257–304, 1986. doi: 10.1016/0304-4157(86)90002-X. [DOI] [PubMed] [Google Scholar]
- 27.International Organization of Standardization. Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity; German version EN ISO 10993‐5:2009. ISO 10993‐52009. https://www.iso.org/standard/36406.html. [2021 Aug 24].
- 28.Thelen T, Hao Y, Medeiros AI, Curtis JL, Serezani CH, Kobzik L, Harris LH, Aronoff DM. The class A scavenger receptor, macrophage receptor with collagenous structure, is the major phagocytic receptor for Clostridium sordellii expressed by human decidual macrophages. J Immunol 185: 4328–4335, 2010. doi: 10.4049/jimmunol.1000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Rooijen N, van Nieuwmegen R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res 238: 355–358, 1984. doi: 10.1007/BF00217308. [DOI] [PubMed] [Google Scholar]
- 30.Cote CK, Van Rooijen N, Welkos SL. Roles of macrophages and neutrophils in the early host response to Bacillus anthracis spores in a mouse model of infection. Infect Immun 74: 469–480, 2006. doi: 10.1128/IAI.74.1.469-480.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika 52: 591–611, 1965. doi: 10.2307/2333709. [DOI] [Google Scholar]
- 32.Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall, Inc., 1974, p. 157. [Google Scholar]
- 33.Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, Kohli M, Thibodeau SN, Boardman L, Wang L. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 14: 319, 2013. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol 165: 77–84, 2013. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 11: 108, 2011. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montaner LJ, da Silva RP, Sun J, Sutterwala S, Hollinshead M, Vaux D, Gordon S. Type 1 and type 2 cytokine regulation of macrophage endocytosis: differential activation by IL-4/IL-13 as opposed to IFN-γ or IL-10. J Immunol 162: 4606–4613, 1999. [PubMed] [Google Scholar]
- 37.Doi T, Higashino K, Kurihara Y, Wada Y, Miyazaki T, Nakamura H, Uesugi S, Imanishi T, Kawabe Y, Itakura H. Charged collagen structure mediates the recognition of negatively charged macromolecules by macrophage scavenger receptors. J Biol Chem 268: 2126–2133, 1993. doi: 10.1016/S0021-9258(18)53971-5. [DOI] [PubMed] [Google Scholar]
- 38.Sancho-Albero M, Navascués N, Mendoza G, Sebastián V, Arruebo M, Martín-Duque P, Santamaría J. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J Nanobiotechnology 17: 16, 2019. doi: 10.1186/s12951-018-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett 371: 48–61, 2016. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Kyakulaga AH, Wilcher SA, Gupta RC. Milk exosomes - natural nanoparticles for siRNA delivery. Cancer Lett 449: 186–195, 2019. doi: 10.1016/j.canlet.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Takov K, Yellon DM, Davidson SM. Confounding factors in vesicle uptake studies using fluorescent lipophilic membrane dyes. J Extracell Vesicles 6: 1388731, 2017. doi: 10.1080/20013078.2017.1388731. [DOI] [PMC free article] [PubMed] [Google Scholar]