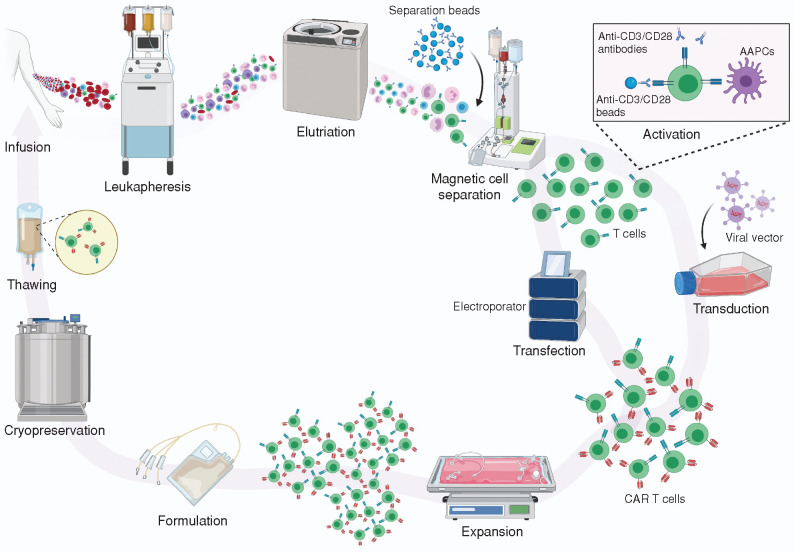
Figure 1.
The manufacturing process of clinical scale autologous CAR T cell therapies. The process starts with the isolation of PBMCs, collected either from whole blood phlebotomy or, more commonly, through a leukapheresis procedure in hospital apheresis units accredited by organizations such as the Foundation for the Accreditation of Cellular Therapy (FACT) and the Joint Accreditation Committee of ISCT-EBMT (JACIE). The PBMCs can be either cryopreserved locally and then shipped to centralized manufacturing facilities or transferred as fresh products to these centers. Often, density gradient centrifugation or elutriation is performed to reduce unwanted contaminating cells such as granulocytes, red blood cells, and platelets. The selection or depletion of specific T-cell types within the PBMCs is then performed. In some protocols, the cells are enriched for CD3+ T cells prior to or concurrent with their activation, at which point the cells are genetically modified using viral vectors or other nonviral gene delivery methods to express the CAR. The cells are then expanded in the presence of cytokines to a dose suitable for patient administration. In most protocols, the expanded cells are then formulated in an appropriate cryopreservation medium containing dimethyl sulfoxide (DMSO) and cryopreserved. After passing quality control and quality assurance lot release requirements, the gene-modified cells are shipped cryopreserved using a validated liquid nitrogen shipper to treatment locations, where the product is thawed under controlled conditions and infused into patients. AAPCs, artifical antigen-presenting cells. Created with BioRender.com.